Recall involves the complete removal of a medical device from the market due to serious safety risks or defects that may cause harm to patients. Field Safety Corrective Action (FSCA) addresses less severe issues by implementing corrective measures such as device modifications or updates without removing the product entirely. Both processes aim to ensure patient safety and regulatory compliance by mitigating risks associated with medical devices.
Table of Comparison
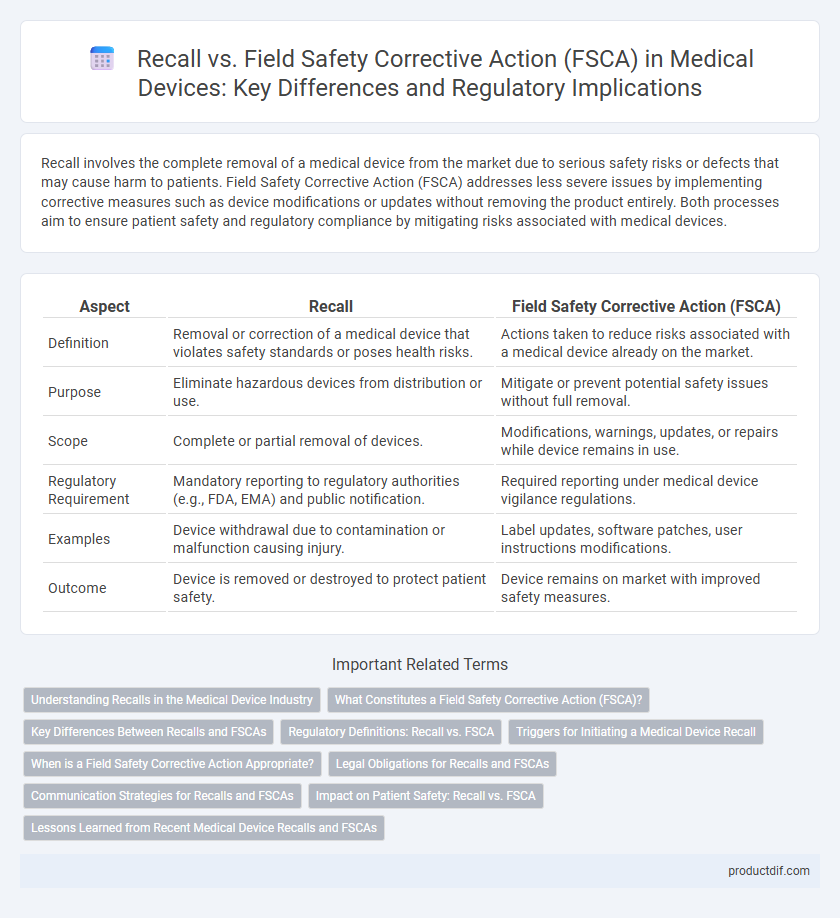
| Aspect | Recall | Field Safety Corrective Action (FSCA) |
|---|---|---|
| Definition | Removal or correction of a medical device that violates safety standards or poses health risks. | Actions taken to reduce risks associated with a medical device already on the market. |
| Purpose | Eliminate hazardous devices from distribution or use. | Mitigate or prevent potential safety issues without full removal. |
| Scope | Complete or partial removal of devices. | Modifications, warnings, updates, or repairs while device remains in use. |
| Regulatory Requirement | Mandatory reporting to regulatory authorities (e.g., FDA, EMA) and public notification. | Required reporting under medical device vigilance regulations. |
| Examples | Device withdrawal due to contamination or malfunction causing injury. | Label updates, software patches, user instructions modifications. |
| Outcome | Device is removed or destroyed to protect patient safety. | Device remains on market with improved safety measures. |
Understanding Recalls in the Medical Device Industry
Recalls in the medical device industry occur when a product is found to pose a risk to patient safety or fails to meet regulatory standards, requiring removal or correction. Field Safety Corrective Actions (FSCAs) involve specific measures taken to reduce risk associated with a device still in use, such as design changes or updates, without necessitating a full market withdrawal. Understanding these distinctions helps manufacturers and healthcare providers ensure compliance with regulatory bodies like the FDA and maintain patient safety through appropriate risk mitigation strategies.
What Constitutes a Field Safety Corrective Action (FSCA)?
A Field Safety Corrective Action (FSCA) involves any measure taken by a medical device manufacturer to reduce health risks associated with a device already on the market, such as modifications, updates, or notifications to users. It includes corrective actions like device repairs, software updates, or instructions for safe use without necessarily withdrawing the product entirely. FSCA is distinct from recalls by focusing on mitigating safety hazards while maintaining device availability when possible.
Key Differences Between Recalls and FSCAs
Recalls involve the complete removal or correction of a medical device from the market due to safety risks or regulatory non-compliance, ensuring patient safety and regulatory adherence. Field Safety Corrective Actions (FSCAs) are specific measures taken to address less severe issues, such as device malfunctions or labeling errors, without withdrawing the product entirely. The primary difference lies in the scope and severity of the intervention, where recalls demand full market retrieval, and FSCAs focus on targeted corrections or notifications to users.
Regulatory Definitions: Recall vs. FSCA
Recall refers to the removal or correction of a medical device that is in violation of regulatory standards and poses a risk to health, mandated by authorities like the FDA or EMA. Field Safety Corrective Action (FSCA) encompasses any voluntary or mandatory actions taken by the manufacturer to reduce a risk associated with a medical device already on the market, including device modifications, software updates, or user instructions. Regulatory frameworks classify recalls as formal, often public procedures involving device withdrawal, while FSCAs can be less formal, focusing on risk mitigation without necessarily removing the device from use.
Triggers for Initiating a Medical Device Recall
Triggers for initiating a medical device recall typically include identification of significant safety risks such as device malfunctions causing harm, failure to meet regulatory standards, or contamination during manufacturing. Adverse event reports, customer complaints, and internal quality audits serve as primary indicators prompting immediate corrective measures. Regulatory authorities may also mandate recalls based on post-market surveillance data revealing potential threats to patient safety.
When is a Field Safety Corrective Action Appropriate?
A Field Safety Corrective Action (FSCA) is appropriate when a medical device presents a risk that can be managed without removing the product from the market, such as issues requiring updates, labeling changes, or user instructions to mitigate potential hazards. FSCA targets non-life-threatening problems that do not necessitate a full recall but still demand corrective measures to ensure patient safety and regulatory compliance. Regulatory bodies like the FDA and the European Medicines Agency require manufacturers to implement FSCA when a product correction or removal is necessary to prevent harm while maintaining device availability.
Legal Obligations for Recalls and FSCAs
Legal obligations for medical device recalls require manufacturers to promptly notify regulatory authorities and affected users about safety risks, ensuring traceability and corrective actions to mitigate harm. Field Safety Corrective Actions (FSCAs) mandate manufacturers to implement measures such as device modifications, user instructions, or warnings without necessarily removing the product from the market, under strict regulatory oversight. Compliance with these obligations is governed by regulations like the EU Medical Device Regulation (MDR) 2017/745 and the FDA guidelines, emphasizing timely communication, documentation, and risk management to protect patient safety.
Communication Strategies for Recalls and FSCAs
Effective communication strategies for Recall and Field Safety Corrective Actions (FSCAs) in medical devices emphasize timely, clear, and transparent stakeholder notifications, including healthcare providers, regulatory bodies, and patients. Utilizing multiple communication channels such as direct emails, regulatory databases, and public notices ensures comprehensive awareness and prompt corrective measures. Detailed messaging that specifies the risk, affected device model, and required actions enhances compliance and patient safety.
Impact on Patient Safety: Recall vs. FSCA
Recalls in medical devices typically indicate a significant risk to patient safety, often involving device malfunctions or defects that can lead to serious injury or death. Field Safety Corrective Actions (FSCA) address less critical issues, focusing on mitigation steps such as software updates or labeling changes that reduce potential risks without immediate danger. The severity and immediacy of patient safety impact distinguish recalls from FSCAs, with recalls demanding urgent removal or repair and FSCAs providing targeted corrections to ensure ongoing device safety.
Lessons Learned from Recent Medical Device Recalls and FSCAs
Recent medical device recalls and Field Safety Corrective Actions (FSCAs) highlight critical lessons in risk management and regulatory compliance. Effective root cause analysis and prompt communication with regulatory authorities are essential to minimize patient harm and maintain trust. Data shows that integrating real-time monitoring systems can significantly reduce the frequency and impact of such corrective actions.
Recall vs Field Safety Corrective Action Infographic
 productdif.com
productdif.com