Recall procedures involve the systematic removal or correction of a medical device from the market due to safety concerns or regulatory non-compliance, ensuring patient protection and regulatory adherence. Field safety corrective actions (FSCA) address specific risks by implementing targeted measures such as device modification, enhanced warnings, or instructions without withdrawing the product entirely. Both processes are critical for maintaining medical device safety but differ in scope and execution depending on the severity of the identified issue.
Table of Comparison
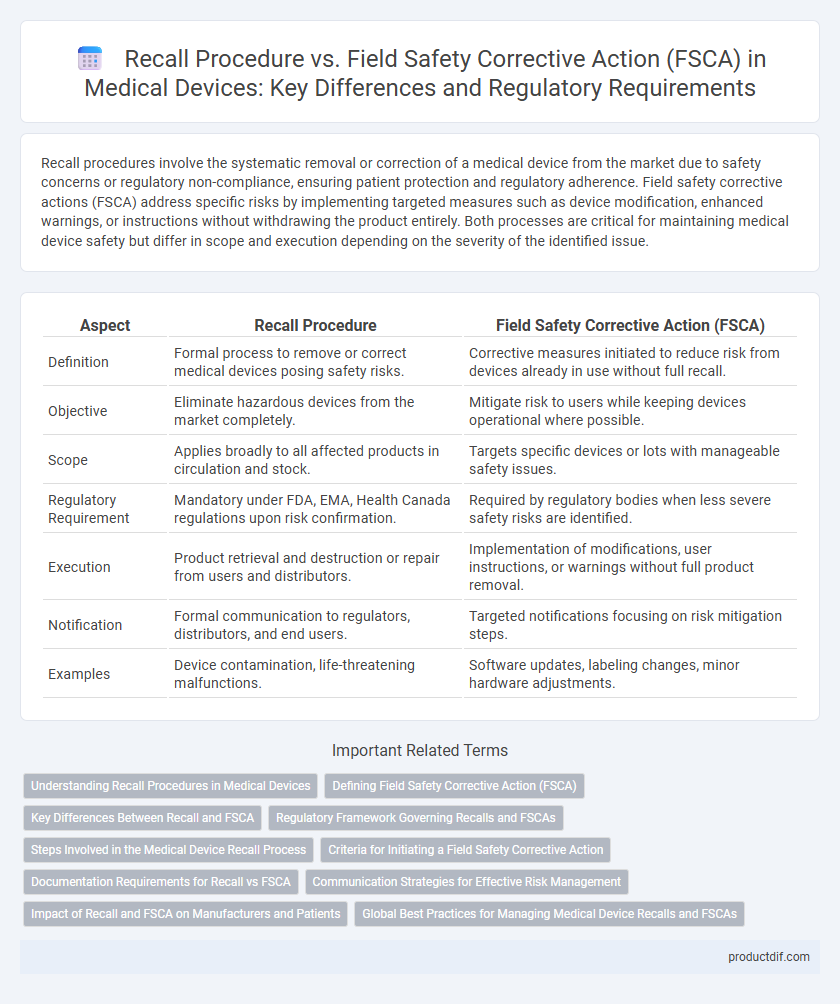
| Aspect | Recall Procedure | Field Safety Corrective Action (FSCA) |
|---|---|---|
| Definition | Formal process to remove or correct medical devices posing safety risks. | Corrective measures initiated to reduce risk from devices already in use without full recall. |
| Objective | Eliminate hazardous devices from the market completely. | Mitigate risk to users while keeping devices operational where possible. |
| Scope | Applies broadly to all affected products in circulation and stock. | Targets specific devices or lots with manageable safety issues. |
| Regulatory Requirement | Mandatory under FDA, EMA, Health Canada regulations upon risk confirmation. | Required by regulatory bodies when less severe safety risks are identified. |
| Execution | Product retrieval and destruction or repair from users and distributors. | Implementation of modifications, user instructions, or warnings without full product removal. |
| Notification | Formal communication to regulators, distributors, and end users. | Targeted notifications focusing on risk mitigation steps. |
| Examples | Device contamination, life-threatening malfunctions. | Software updates, labeling changes, minor hardware adjustments. |
Understanding Recall Procedures in Medical Devices
Recall procedures in medical devices involve systematically identifying, retrieving, and correcting or removing products that pose safety risks or do not comply with regulatory standards. Field safety corrective actions (FSCA) are specific measures implemented to reduce the risk posed by faulty devices still in use, often issued alongside or as part of recalls. Understanding these processes ensures timely mitigation of hazards, compliance with agencies like the FDA or EMA, and protection of patient health.
Defining Field Safety Corrective Action (FSCA)
Field Safety Corrective Action (FSCA) refers to measures initiated by medical device manufacturers or regulatory authorities to reduce the risk of harm associated with a device already on the market. FSCA includes actions such as device modifications, software updates, or user instructions designed to mitigate identified safety issues without necessarily removing the device from use. This proactive approach targets the improvement of device safety while maintaining availability to patients and healthcare providers.
Key Differences Between Recall and FSCA
Recall procedures involve the complete removal of a defective or potentially harmful medical device from the market or user possession, ensuring patient safety by withdrawing all affected products. Field Safety Corrective Actions (FSCAs) focus on mitigating risks through repairs, modifications, or providing updated instructions without necessarily removing the device entirely. Key differences include the scope of action, with recalls being more comprehensive and often regulatory-mandated, while FSCAs are targeted measures addressing specific safety issues without full product withdrawal.
Regulatory Framework Governing Recalls and FSCAs
The regulatory framework governing medical device recalls and Field Safety Corrective Actions (FSCAs) is primarily defined by authorities such as the FDA in the United States and the European Medicines Agency (EMA) in the EU under the Medical Device Regulation (MDR) 2017/745. Recalls involve removing defective devices from the market due to safety risks, while FSCAs address corrective actions to mitigate risk without full removal. Compliance with stringent reporting requirements, risk assessment protocols, and timely communication to health authorities forms the core of regulatory oversight for both recalls and FSCAs.
Steps Involved in the Medical Device Recall Process
The medical device recall process involves identifying the defect, notifying regulatory bodies, and informing healthcare providers and patients to mitigate risks. Field safety corrective actions focus on addressing specific safety concerns through targeted corrections such as product modifications or instructions for use updates. Both procedures require documentation, root cause analysis, and post-action monitoring to ensure the effectiveness of the corrective measures and compliance with regulatory standards.
Criteria for Initiating a Field Safety Corrective Action
Criteria for initiating a Field Safety Corrective Action (FSCA) in medical devices include identifying a significant risk to patient or user safety that cannot be mitigated through routine measures. The decision hinges on evidence of non-conformity or a defect that may cause serious injury or death, requiring immediate communication to regulatory authorities and end-users. FSCA protocols emphasize timely risk assessment, effective traceability of affected devices, and clear instructions for corrective measures to prevent harm.
Documentation Requirements for Recall vs FSCA
Recall procedures require comprehensive documentation including root cause analysis, risk assessment, product identification, and detailed corrective actions to ensure traceability and regulatory compliance. Field Safety Corrective Actions (FSCA) documentation focuses on prompt communication to healthcare professionals, beneficiary notification, and effectiveness checks, emphasizing patient safety and ongoing monitoring. Both processes mandate clear records but differ in scope: recalls necessitate exhaustive evidence for product removal, while FSCA centers on minimizing risks during product use.
Communication Strategies for Effective Risk Management
Communication strategies in Medical Device Recall procedures emphasize direct, clear notifications to healthcare providers and patients, ensuring immediate awareness of identified risks. In Field Safety Corrective Actions, targeted communication includes detailed guidance on device modifications or usage adjustments, fostering compliance and minimizing harm. Both approaches prioritize timely, evidence-based messaging to uphold patient safety and regulatory compliance during risk management.
Impact of Recall and FSCA on Manufacturers and Patients
Recall procedures and Field Safety Corrective Actions (FSCA) significantly impact medical device manufacturers by imposing regulatory scrutiny, financial burdens, and potential reputational damage. For patients, recalls and FSCAs ensure the removal or correction of devices that pose safety risks, thereby safeguarding public health and reducing the incidence of adverse events. Both processes emphasize prompt communication, risk assessment, and traceability to minimize harm and facilitate effective resolution.
Global Best Practices for Managing Medical Device Recalls and FSCAs
Global best practices for managing medical device recalls and Field Safety Corrective Actions (FSCAs) emphasize the importance of a structured communication strategy with regulatory bodies such as the FDA, EMA, and other health authorities. Effective traceability systems and risk assessment protocols enable precise identification and mitigation of potential hazards, ensuring patient safety and compliance across diverse markets. Implementation of robust post-market surveillance and timely corrective measures reduces operational disruptions and reinforces trust in device safety standards.
Recall procedure vs Field safety corrective action Infographic
 productdif.com
productdif.com