Reprocessing medical devices involves thorough cleaning, sterilization, and testing to ensure safety for multiple uses, significantly reducing medical waste and lowering healthcare costs. Single-use devices eliminate the risk of cross-contamination but generate more waste and often have higher long-term costs due to the need for continuous purchases. Healthcare facilities must balance infection control priorities with environmental and economic considerations when choosing between reprocessing and single-use options.
Table of Comparison
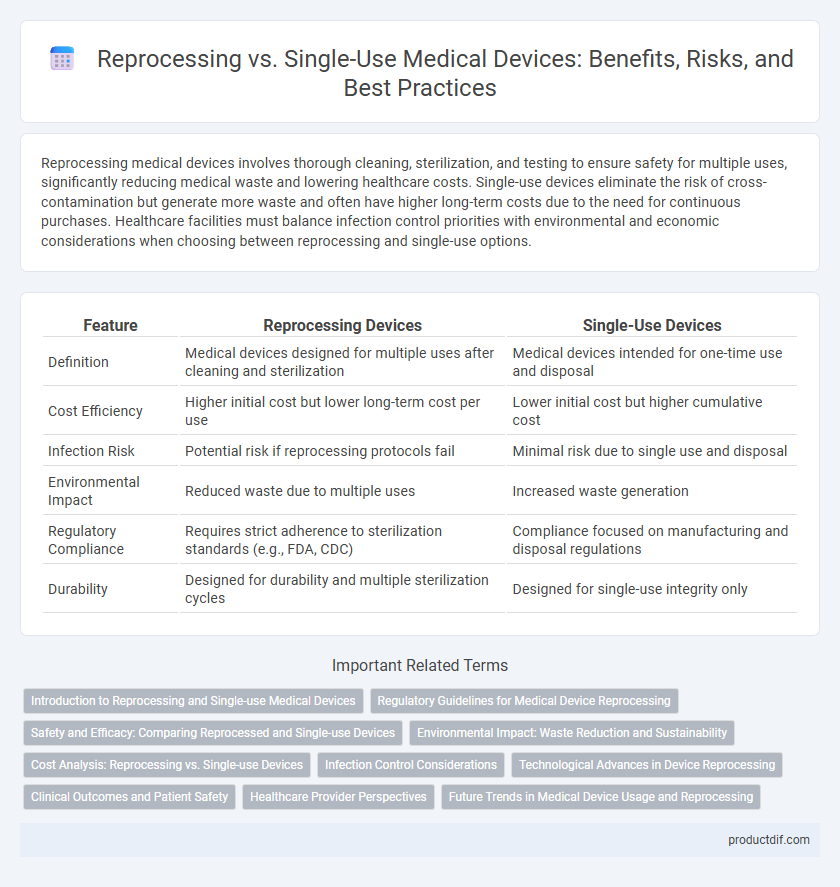
| Feature | Reprocessing Devices | Single-Use Devices |
|---|---|---|
| Definition | Medical devices designed for multiple uses after cleaning and sterilization | Medical devices intended for one-time use and disposal |
| Cost Efficiency | Higher initial cost but lower long-term cost per use | Lower initial cost but higher cumulative cost |
| Infection Risk | Potential risk if reprocessing protocols fail | Minimal risk due to single use and disposal |
| Environmental Impact | Reduced waste due to multiple uses | Increased waste generation |
| Regulatory Compliance | Requires strict adherence to sterilization standards (e.g., FDA, CDC) | Compliance focused on manufacturing and disposal regulations |
| Durability | Designed for durability and multiple sterilization cycles | Designed for single-use integrity only |
Introduction to Reprocessing and Single-use Medical Devices
Reprocessing medical devices involves cleaning, sterilizing, and testing reusable instruments to ensure patient safety and maintain device integrity, reducing healthcare costs and environmental waste. Single-use medical devices are designed for one-time use, minimizing the risk of cross-contamination but generating more medical waste and higher long-term expenses. Effective decision-making between reprocessing and single-use options depends on device type, regulatory guidelines, and infection control protocols.
Regulatory Guidelines for Medical Device Reprocessing
Regulatory guidelines for medical device reprocessing emphasize strict adherence to validated cleaning, disinfection, and sterilization protocols to ensure patient safety and device functionality. Agencies such as the FDA and MDR mandate comprehensive documentation, risk assessments, and routine audits to verify that reprocessed devices meet original performance standards. Compliance with these regulations reduces the risk of infection transmission and supports sustainable healthcare practices by enabling safe reuse of approved devices.
Safety and Efficacy: Comparing Reprocessed and Single-use Devices
Reprocessed medical devices undergo rigorous sterilization and quality checks to ensure safety and efficacy comparable to single-use devices, often demonstrating equivalent infection control and functional reliability. Regulatory agencies, including the FDA, mandate strict guidelines for reprocessing that safeguard patient outcomes while enabling cost-effective reuse. Studies consistently reveal that reprocessed devices maintain clinical performance standards without increasing the risk of device-related complications when processed according to validated protocols.
Environmental Impact: Waste Reduction and Sustainability
Reprocessing medical devices significantly reduces biomedical waste by enabling multiple uses of high-quality materials, thereby minimizing the environmental footprint compared to single-use devices. Sustainable practices in reprocessing lower landfill contributions and decrease the demand for raw materials, promoting resource conservation and energy efficiency. Lifecycle assessments indicate that reprocessed devices contribute to substantial waste reduction and support healthcare facilities' sustainability goals.
Cost Analysis: Reprocessing vs. Single-use Devices
Cost analysis of medical devices reveals that reprocessing single-use devices often reduces overall expenses by enabling multiple uses per unit, lowering procurement costs. However, reprocessing requires investment in sterilization equipment, labor, and quality control, which may vary depending on device complexity and hospital volume. Single-use devices eliminate reprocessing costs but involve higher recurring purchase expenses and increased medical waste management costs.
Infection Control Considerations
Reprocessing medical devices involves thorough cleaning, disinfection, and sterilization to prevent cross-contamination and ensure patient safety, but it carries risks of residual biofilm and incomplete sterilization. Single-use devices eliminate the risk of cross-infection by being disposed of after one procedure, reducing concerns about microbial contamination and biofilm formation. Infection control protocols must weigh the benefits of reprocessing cost savings against the potential for pathogen transmission inherent to device reuse.
Technological Advances in Device Reprocessing
Technological advances in device reprocessing have significantly enhanced sterilization methods, reducing infection risks and increasing the safety of reusable medical devices. Innovations such as low-temperature plasma sterilization and advanced automated cleaning systems improve efficiency while preserving device integrity. These developments support cost-effective healthcare by extending device lifespan without compromising clinical performance.
Clinical Outcomes and Patient Safety
Reprocessing medical devices demands stringent sterilization protocols to minimize infection risks and ensure clinical outcomes comparable to single-use devices. Studies indicate that when reprocessing adheres to regulatory standards, patient safety remains uncompromised, with incidence rates of device-related complications paralleling those of disposable counterparts. However, lapses in cleaning and sterilization can elevate risks of cross-contamination, underscoring the necessity of rigorous quality control in reprocessing workflows.
Healthcare Provider Perspectives
Healthcare providers weigh the benefits of reprocessing medical devices against the convenience of single-use products, emphasizing patient safety, cost-effectiveness, and environmental impact. Reprocessing offers reduced medical waste and potential cost savings but requires stringent adherence to sterilization protocols to prevent infection risks. Single-use devices often minimize cross-contamination concerns and simplify workflow, though they can contribute to higher operational costs and increased environmental burden.
Future Trends in Medical Device Usage and Reprocessing
Future trends in medical device usage emphasize increased adoption of advanced sterilization technologies and automated reprocessing systems to enhance safety and efficiency. Innovations in material science are driving the development of reusable devices with improved durability, reducing environmental impact compared to single-use products. Regulatory frameworks are evolving to support sustainable practices while maintaining stringent infection control standards in clinical settings.
Reprocessing vs Single-use Infographic
 productdif.com
productdif.com