Device History Files (DHF) document the design and development process of a medical device, capturing detailed records such as design inputs, specifications, verification, and validation activities. Device Master Records (DMR) compile all necessary information for manufacturing the device, including production procedures, quality assurance protocols, and packaging instructions. Maintaining accurate DHF and DMR ensures compliance with regulatory standards and facilitates traceability throughout the product lifecycle.
Table of Comparison
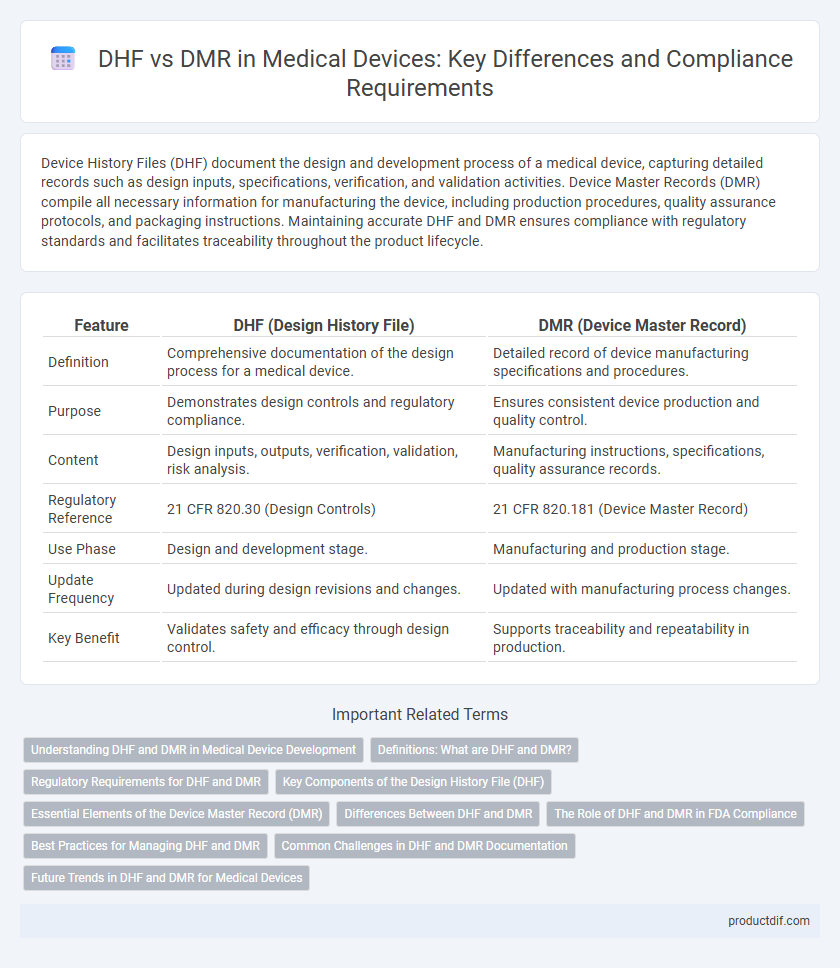
| Feature | DHF (Design History File) | DMR (Device Master Record) |
|---|---|---|
| Definition | Comprehensive documentation of the design process for a medical device. | Detailed record of device manufacturing specifications and procedures. |
| Purpose | Demonstrates design controls and regulatory compliance. | Ensures consistent device production and quality control. |
| Content | Design inputs, outputs, verification, validation, risk analysis. | Manufacturing instructions, specifications, quality assurance records. |
| Regulatory Reference | 21 CFR 820.30 (Design Controls) | 21 CFR 820.181 (Device Master Record) |
| Use Phase | Design and development stage. | Manufacturing and production stage. |
| Update Frequency | Updated during design revisions and changes. | Updated with manufacturing process changes. |
| Key Benefit | Validates safety and efficacy through design control. | Supports traceability and repeatability in production. |
Understanding DHF and DMR in Medical Device Development
Design History File (DHF) documents the design process, capturing all specifications, design inputs, outputs, and verification activities to ensure regulatory compliance in medical device development. Device Master Record (DMR) contains the comprehensive set of instructions, drawings, and records required for manufacturing the finished medical device consistently and according to design. Understanding the distinction between DHF and DMR is crucial for aligning design control with production control, facilitating quality management and FDA submissions.
Definitions: What are DHF and DMR?
The Design History File (DHF) is a comprehensive compilation of records documenting the design development process of a medical device to ensure compliance with regulatory requirements. The Device Master Record (DMR) contains detailed instructions, specifications, and procedures necessary for manufacturing the finished medical device. Together, DHF and DMR form critical components of quality management systems, supporting product consistency and regulatory audits.
Regulatory Requirements for DHF and DMR
Design History File (DHF) and Device Master Record (DMR) are critical regulatory documents in medical device manufacturing, governed by FDA's 21 CFR Part 820. DHF documents the design process, validation, and design controls, ensuring compliance with design control requirements. DMR contains the complete manufacturing specifications, including device drawings, formulations, and instructions, required for consistent production and traceability.
Key Components of the Design History File (DHF)
The Design History File (DHF) documents the entire design process of a medical device, including design inputs, design outputs, design reviews, verification and validation activities, and design changes. Key components within the DHF encompass design plans, risk management files, and traceability matrices that link user needs to design specifications. This comprehensive documentation ensures compliance with FDA 21 CFR Part 820 requirements and facilitates regulatory submissions by demonstrating systematic design control.
Essential Elements of the Device Master Record (DMR)
The Device Master Record (DMR) contains comprehensive documentation essential for manufacturing, including device specifications, production processes, quality assurance protocols, and packaging details. Unlike the Design History File (DHF), which tracks design development and changes, the DMR serves as the definitive compilation of records that ensures consistent device production according to regulatory standards. Key elements of the DMR encompass device components, assembly instructions, quality control procedures, and labeling requirements critical for compliance with FDA regulations.
Differences Between DHF and DMR
The Design History File (DHF) documents the design and development process of a medical device, providing evidence of compliance with design controls throughout the product lifecycle. In contrast, the Device Master Record (DMR) contains the complete manufacturing specifications, including device specifications, production processes, quality assurance procedures, and packaging requirements. While DHF focuses on design validation and verification, the DMR ensures consistent production and quality control for each manufactured device.
The Role of DHF and DMR in FDA Compliance
The Design History File (DHF) documents the design process, ensuring that medical devices meet FDA regulatory requirements through thorough design verification and validation records. The Device Master Record (DMR) contains detailed device specifications, production processes, and quality assurance procedures vital for FDA compliance during manufacturing and post-market surveillance. Together, the DHF and DMR form integral parts of the FDA's Quality System Regulation (QSR), facilitating traceability and demonstrating adherence to 21 CFR Part 820 standards.
Best Practices for Managing DHF and DMR
Effective management of the Design History File (DHF) and Device Master Record (DMR) is critical for medical device compliance and quality assurance. Best practices involve maintaining comprehensive and up-to-date documentation within the DHF to capture the design process, design changes, and verification activities, while ensuring the DMR accurately reflects the finished device specifications, manufacturing instructions, and quality assurance procedures. Leveraging integrated electronic document management systems enhances traceability, facilitates regulatory audits, and supports continuous product improvement throughout the device lifecycle.
Common Challenges in DHF and DMR Documentation
DHF (Design History File) and DMR (Device Master Record) documentation both face challenges in maintaining accuracy, traceability, and compliance with FDA regulations. Common issues include inconsistent data entries, version control problems, and inadequate linkage between design inputs and outputs. Ensuring thorough, audit-ready records requires robust document management systems and rigorous cross-functional collaboration.
Future Trends in DHF and DMR for Medical Devices
Future trends in Design History File (DHF) and Device Master Record (DMR) for medical devices emphasize increased digitalization and integration of advanced software tools to enhance traceability and compliance. Emerging technologies such as blockchain and AI-driven analytics are expected to improve data integrity and streamline regulatory submissions. Enhanced interoperability between DHF and DMR systems will support real-time updates and facilitate agile product development cycles in the medical device industry.
DHF vs DMR Infographic
 productdif.com
productdif.com