Implantable medical devices are surgically placed inside the body to provide continuous monitoring or therapy, offering long-term solutions for chronic conditions. Non-implantable devices remain external, allowing for easy removal and adjustments, ideal for short-term use or diagnostic purposes. The choice between implantable and non-implantable devices depends on patient needs, treatment duration, and risk factors.
Table of Comparison
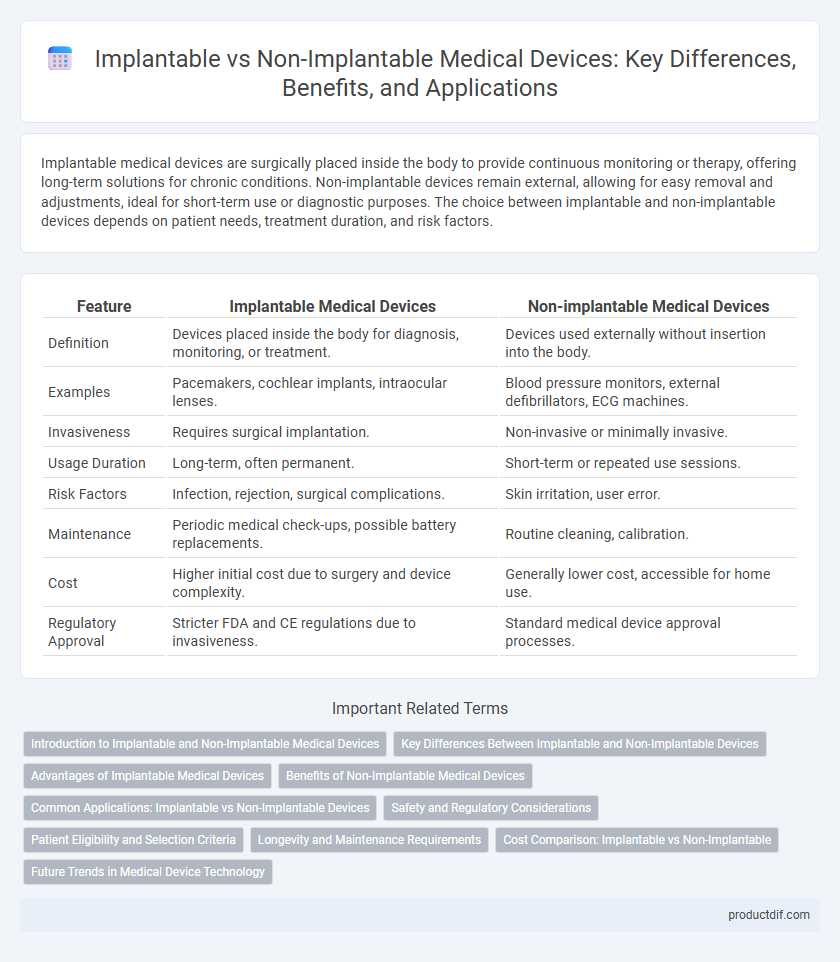
| Feature | Implantable Medical Devices | Non-implantable Medical Devices |
|---|---|---|
| Definition | Devices placed inside the body for diagnosis, monitoring, or treatment. | Devices used externally without insertion into the body. |
| Examples | Pacemakers, cochlear implants, intraocular lenses. | Blood pressure monitors, external defibrillators, ECG machines. |
| Invasiveness | Requires surgical implantation. | Non-invasive or minimally invasive. |
| Usage Duration | Long-term, often permanent. | Short-term or repeated use sessions. |
| Risk Factors | Infection, rejection, surgical complications. | Skin irritation, user error. |
| Maintenance | Periodic medical check-ups, possible battery replacements. | Routine cleaning, calibration. |
| Cost | Higher initial cost due to surgery and device complexity. | Generally lower cost, accessible for home use. |
| Regulatory Approval | Stricter FDA and CE regulations due to invasiveness. | Standard medical device approval processes. |
Introduction to Implantable and Non-Implantable Medical Devices
Implantable medical devices, such as pacemakers and cochlear implants, are surgically placed inside the body to monitor or treat medical conditions continuously. Non-implantable devices include external tools like blood glucose monitors and portable oxygen concentrators used temporarily or intermittently for patient care. Understanding the differences between these devices is crucial for selecting appropriate medical solutions based on patient needs, treatment goals, and risk factors.
Key Differences Between Implantable and Non-Implantable Devices
Implantable medical devices, such as pacemakers and cochlear implants, are surgically placed inside the body to provide continuous monitoring or therapeutic functions, while non-implantable devices like external monitors and infusion pumps remain outside the body for temporary or intermittent use. Implantable devices typically offer long-term intervention, advanced biocompatibility, and are designed to function reliably in a physiological environment, whereas non-implantable devices prioritize ease of access, adjustability, and lower risk of infection. The choice between implantable and non-implantable devices depends on clinical needs, duration of treatment, invasiveness, and patient lifestyle considerations.
Advantages of Implantable Medical Devices
Implantable medical devices offer continuous and accurate monitoring or therapeutic intervention directly within the body, improving patient compliance and reducing the risk of infection associated with external devices. These devices provide real-time data collection, enabling personalized treatment adjustments and enhanced disease management. Their discreet nature and long-term functionality also minimize lifestyle disruption, promoting better overall health outcomes.
Benefits of Non-Implantable Medical Devices
Non-implantable medical devices offer significant benefits including lower risk of infection and easier maintenance since they do not require surgical insertion. Their non-invasive nature allows for quicker patient recovery times and immediate discontinuation if adverse effects occur. These devices also provide greater flexibility for diagnostic and therapeutic applications, facilitating regular updates and adjustments without additional procedures.
Common Applications: Implantable vs Non-Implantable Devices
Implantable medical devices, such as pacemakers, cochlear implants, and orthopedic prosthetics, are primarily used for long-term therapeutic interventions within the body. Non-implantable devices like blood pressure monitors, external defibrillators, and diagnostic imaging equipment serve temporary or external monitoring and treatment purposes. The choice between implantable and non-implantable devices depends on factors such as treatment duration, invasiveness, and patient-specific medical needs.
Safety and Regulatory Considerations
Implantable medical devices undergo rigorous safety evaluations and must comply with stringent regulatory standards such as FDA's Class III premarket approval or MDR's Class III classification due to their direct interaction with internal body tissues. Non-implantable devices typically face less stringent regulatory pathways, often classified as Class I or II, requiring demonstration of safety primarily through performance testing and risk management. Both categories demand adherence to international standards like ISO 13485 to ensure quality management systems, but implantable devices require more comprehensive clinical data to address biocompatibility and long-term safety risks.
Patient Eligibility and Selection Criteria
Patient eligibility for implantable medical devices requires comprehensive assessment of anatomical suitability, underlying health conditions, and risk factors for infection or rejection. Non-implantable devices are typically selected for patients with temporary needs or contraindications to surgery, emphasizing ease of use and minimal invasiveness. Selection criteria incorporate patient age, comorbidities, lifestyle requirements, and the intended duration of therapy to optimize clinical outcomes.
Longevity and Maintenance Requirements
Implantable medical devices typically offer longer-term functionality with lifespans ranging from 5 to 20 years, reducing the need for frequent replacements but requiring surgical procedures for maintenance or removal. Non-implantable devices usually have shorter lifespans of 1 to 5 years and necessitate regular maintenance such as battery replacement or calibration to ensure accurate performance. The choice between implantable and non-implantable devices depends heavily on patient condition, device purpose, and balancing longevity against maintenance complexity.
Cost Comparison: Implantable vs Non-Implantable
Implantable medical devices typically incur higher initial costs due to advanced materials, surgical implantation, and longer-term maintenance requirements, while non-implantable devices often have lower upfront expenses and reduced healthcare facility involvement. The total cost of implantable devices may include surgery fees, follow-up care, and potential revision procedures, contrasting with non-implantable devices which usually involve straightforward usage and replacement costs. Cost-effectiveness analysis favors non-implantable devices for short-term applications, whereas implantable devices justify higher costs through improved patient compliance and long-term therapeutic benefits.
Future Trends in Medical Device Technology
Future trends in medical device technology reveal a growing convergence between implantable and non-implantable devices through advancements in wireless connectivity, miniaturization, and biocompatible materials. Innovations such as smart implants equipped with real-time monitoring sensors and AI-driven data analytics enhance personalized patient care, while non-implantable wearables integrate seamlessly with cloud platforms for continuous health tracking. Emerging fields like energy harvesting and nanotechnology further bridge the gap, enabling devices to operate autonomously and with extended lifespans, shaping the next generation of medical interventions.
Implantable vs Non-implantable Infographic
 productdif.com
productdif.com