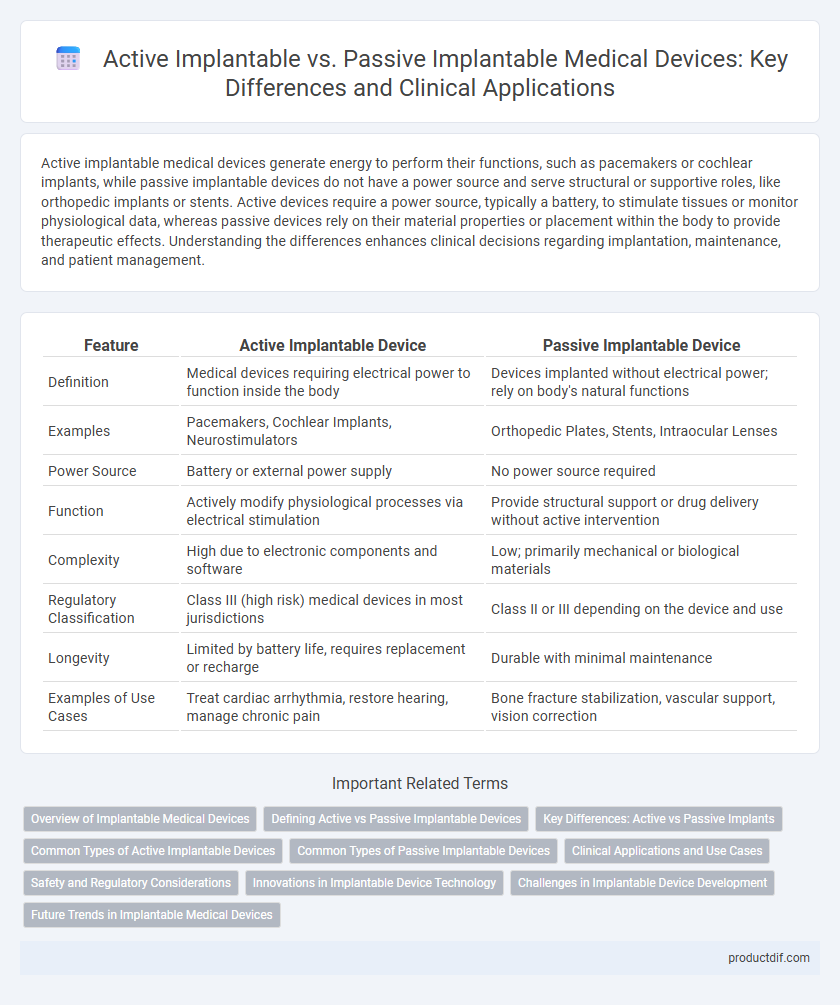
Active implantable medical devices generate energy to perform their functions, such as pacemakers or cochlear implants, while passive implantable devices do not have a power source and serve structural or supportive roles, like orthopedic implants or stents. Active devices require a power source, typically a battery, to stimulate tissues or monitor physiological data, whereas passive devices rely on their material properties or placement within the body to provide therapeutic effects. Understanding the differences enhances clinical decisions regarding implantation, maintenance, and patient management.
Table of Comparison
| Feature | Active Implantable Device | Passive Implantable Device |
|---|---|---|
| Definition | Medical devices requiring electrical power to function inside the body | Devices implanted without electrical power; rely on body's natural functions |
| Examples | Pacemakers, Cochlear Implants, Neurostimulators | Orthopedic Plates, Stents, Intraocular Lenses |
| Power Source | Battery or external power supply | No power source required |
| Function | Actively modify physiological processes via electrical stimulation | Provide structural support or drug delivery without active intervention |
| Complexity | High due to electronic components and software | Low; primarily mechanical or biological materials |
| Regulatory Classification | Class III (high risk) medical devices in most jurisdictions | Class II or III depending on the device and use |
| Longevity | Limited by battery life, requires replacement or recharge | Durable with minimal maintenance |
| Examples of Use Cases | Treat cardiac arrhythmia, restore hearing, manage chronic pain | Bone fracture stabilization, vascular support, vision correction |
Overview of Implantable Medical Devices
Active implantable medical devices, such as pacemakers and cochlear implants, require electrical power to perform therapeutic functions, while passive implantable devices like orthopedic pins and stents provide structural support without electrical components. Both categories play critical roles in patient care by restoring or enhancing physiological functions through minimally invasive techniques. Understanding the differences between active and passive implantables aids clinicians in device selection, ensuring optimal treatment outcomes based on the patient's specific clinical needs.
Defining Active vs Passive Implantable Devices
Active implantable devices contain a power source and perform electrical or mechanical functions within the body, such as pacemakers and cochlear implants, enabling continuous therapeutic or diagnostic activity. Passive implantable devices do not have an internal power source and serve primarily structural or supportive roles, like stents or surgical meshes, relying on the body's natural processes to function effectively. The distinction centers on the presence of an internal energy source and active functionality versus inert, non-powered biomedical components.
Key Differences: Active vs Passive Implants
Active implantable medical devices, such as pacemakers and cochlear implants, require an internal power source to function and actively interact with body tissues or organs for therapeutic purposes. In contrast, passive implantable devices, like artificial joints or stents, do not have a power source and serve primarily structural or supportive roles without active physiological intervention. Key differences include the presence of electronic components and energy dependence in active implants, whereas passive implants rely solely on biocompatible materials for integration into the body.
Common Types of Active Implantable Devices
Common types of active implantable devices include pacemakers, cochlear implants, and neurostimulators, which deliver electrical stimulation to regulate bodily functions or restore sensory capabilities. These devices require a power source, typically a battery, to operate and perform tasks such as cardiac rhythm management or hearing restoration. Unlike passive implantable devices that do not generate energy, active implants directly interact with physiological processes for therapeutic effects.
Common Types of Passive Implantable Devices
Common types of passive implantable devices include orthopedic implants, such as joint replacements and bone plates, dental implants, and vascular stents. These devices do not require a power source or electronic components to perform their function, relying instead on biocompatible materials like titanium or ceramics to provide structural support or facilitate tissue integration. Their primary role is to restore function or maintain the integrity of biological structures without active intervention or signal transmission.
Clinical Applications and Use Cases
Active implantable medical devices, such as pacemakers and cochlear implants, deliver electrical stimulation or therapeutic effects directly to target tissues, making them essential in cardiac rhythm management and sensory restoration. Passive implantable devices, including orthopedic screws and dental implants, provide structural support or facilitate healing without active energy delivery, playing a crucial role in musculoskeletal repair and dental rehabilitation. Clinical applications of active implants often involve continuous monitoring and adjustment, while passive implants primarily serve as stable frameworks to enhance natural biological processes.
Safety and Regulatory Considerations
Active implantable medical devices, which rely on electrical energy to perform their intended function, require rigorous safety testing including electromagnetic compatibility and battery reliability to comply with regulatory standards such as the FDA's 21 CFR Part 820 and the EU Medical Device Regulation (MDR 2017/745). Passive implantable devices, lacking electrical components, primarily demand biocompatibility assessments and structural integrity verification to meet ISO 10993 guidelines and ensure patient safety. Both categories must undergo post-market surveillance to detect adverse events, maintain compliance, and support continuous safety monitoring throughout their lifecycle.
Innovations in Implantable Device Technology
Innovations in implantable device technology have significantly advanced both active implantable devices, such as pacemakers and neurostimulators, and passive implantable devices like orthopedic implants and intraocular lenses. Active implantable devices now integrate wireless communication, battery-less energy harvesting, and real-time biometric monitoring, enhancing functionality and patient outcomes. Passive implantable devices benefit from novel biomaterials and surface modifications that improve biocompatibility and reduce infection risks.
Challenges in Implantable Device Development
Active implantable devices, such as pacemakers and neurostimulators, face challenges including power management, biocompatibility, and real-time data processing, while passive implantable devices like orthopedic implants mainly contend with material durability and tissue integration. Both types require rigorous assessment to prevent immune rejection, device failure, and infection risks, complicating long-term reliability and patient safety. Advances in miniaturization, battery technology, and smart biomaterials aim to overcome these hurdles in medical device development.
Future Trends in Implantable Medical Devices
Future trends in implantable medical devices emphasize the convergence of active implantable systems, such as pacemakers and neurostimulators, with advanced sensor technologies and wireless communication for real-time monitoring and personalized therapy. Passive implantable devices, including stents and orthopedic implants, are increasingly incorporating bioactive coatings and smart biomaterials to enhance tissue integration and reduce complications. Innovations in energy harvesting and miniaturization are expected to drive the next generation of both active and passive implants, improving patient outcomes and device longevity.
Active implantable vs Passive implantable Infographic
 productdif.com
productdif.com