Disposable medical devices reduce the risk of cross-contamination and infection by eliminating the need for sterilization between uses. Reusable devices offer cost efficiency and environmental benefits but require rigorous cleaning protocols to maintain patient safety. Choosing between disposable and reusable devices depends on factors such as procedure type, infection control standards, and long-term cost considerations.
Table of Comparison
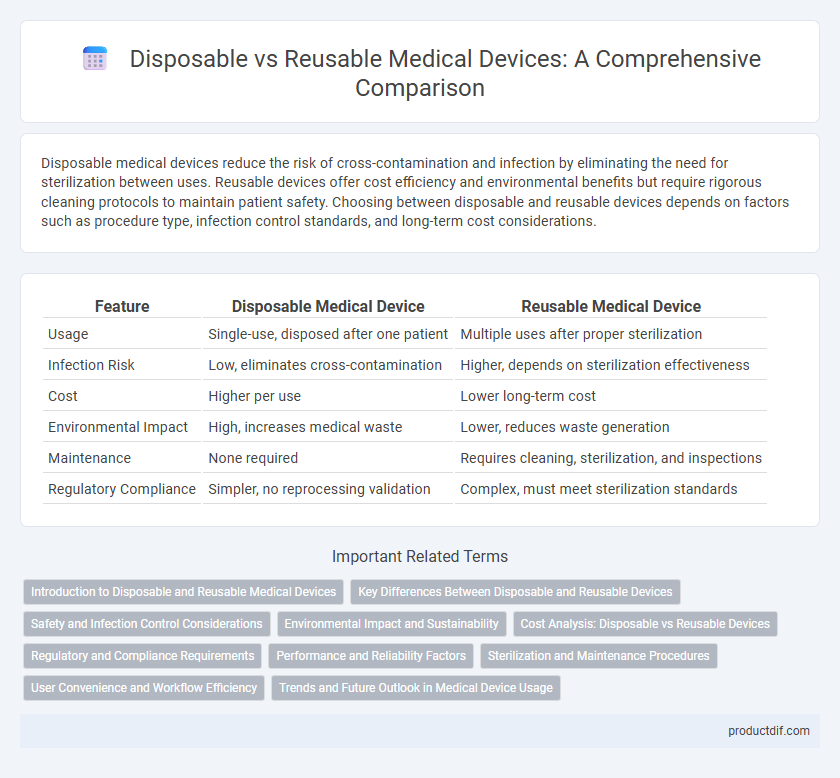
| Feature | Disposable Medical Device | Reusable Medical Device |
|---|---|---|
| Usage | Single-use, disposed after one patient | Multiple uses after proper sterilization |
| Infection Risk | Low, eliminates cross-contamination | Higher, depends on sterilization effectiveness |
| Cost | Higher per use | Lower long-term cost |
| Environmental Impact | High, increases medical waste | Lower, reduces waste generation |
| Maintenance | None required | Requires cleaning, sterilization, and inspections |
| Regulatory Compliance | Simpler, no reprocessing validation | Complex, must meet sterilization standards |
Introduction to Disposable and Reusable Medical Devices
Disposable medical devices are designed for single-use applications, minimizing infection risks and eliminating the need for sterilization between uses, which enhances patient safety. Reusable medical devices require thorough cleaning and sterilization protocols to maintain functionality and prevent cross-contamination, offering cost-efficiency in long-term use. Selection depends on clinical application, infection control standards, and economic considerations in healthcare settings.
Key Differences Between Disposable and Reusable Devices
Disposable medical devices are designed for single-use, reducing the risk of cross-contamination and infection with strict disposal protocols, while reusable devices require thorough sterilization between uses to ensure patient safety. Cost-efficiency varies as disposable devices eliminate reprocessing expenses but generate higher long-term waste management costs, whereas reusable devices incur initial investment and ongoing sterilization costs but lower per-use expenditure. Material composition and durability differ significantly; disposable devices utilize lightweight, cost-effective materials optimized for one-time use, contrasting with robust, durable materials in reusable devices engineered for multiple cycles.
Safety and Infection Control Considerations
Reusable medical devices require stringent sterilization protocols to prevent cross-contamination and healthcare-associated infections, demanding validated cleaning and disinfection methods. Disposable devices eliminate the risks associated with device reprocessing, offering a single-use solution that significantly reduces infection transmission and exposure to bloodborne pathogens. Selecting between disposable and reusable devices must weigh factors such as infection control effectiveness, cost, and environmental impact to optimize patient safety outcomes.
Environmental Impact and Sustainability
Disposable medical devices generate significant medical waste, contributing to environmental pollution and resource depletion due to single-use plastics and non-biodegradable materials. Reusable devices, designed for sterilization and multiple uses, reduce waste output and lower environmental footprints by minimizing raw material consumption and energy use in manufacturing. Implementing sustainable sterilization technologies and using biodegradable materials can further enhance the eco-friendliness of reusable medical devices while maintaining patient safety.
Cost Analysis: Disposable vs Reusable Devices
The cost analysis of disposable versus reusable medical devices reveals significant differences in initial investment and long-term expenses. Disposable devices often incur higher per-unit costs but eliminate sterilization and maintenance fees, reducing infection risks and downtime. Reusable devices require substantial upfront capital and ongoing costs for cleaning, sterilization, and potential repairs, which can impact overall cost-effectiveness depending on usage frequency and healthcare setting.
Regulatory and Compliance Requirements
Disposable medical devices typically face streamlined regulatory pathways focused on sterility assurance and single-use validation, reducing contamination risks. Reusable devices require comprehensive compliance with strict cleaning, sterilization, durability, and functional testing standards to ensure patient safety across multiple uses. Regulatory bodies like the FDA and EU MDR mandate rigorous documentation and validation protocols tailored to these distinct usage categories.
Performance and Reliability Factors
Disposable medical devices offer consistent performance and high reliability by eliminating risks of contamination and device degradation associated with repeated use. Reusable devices, while cost-effective, require stringent cleaning and maintenance protocols to maintain functionality and prevent performance decline over time. Selecting between disposable and reusable options depends on balancing device reliability, sterility assurance, and clinical application demands.
Sterilization and Maintenance Procedures
Disposable medical devices eliminate the need for sterilization and complex maintenance procedures, reducing the risk of cross-contamination and infection. Reusable devices require rigorous sterilization protocols, including autoclaving or chemical disinfection, to ensure patient safety and device integrity. Maintenance of reusable devices demands regular inspection and adherence to manufacturer guidelines to prevent mechanical failure and contamination.
User Convenience and Workflow Efficiency
Disposable medical devices enhance user convenience by eliminating the need for cleaning and sterilization, significantly reducing workflow interruptions and infection risks in clinical settings. Reusable devices, while cost-effective over time, require thorough decontamination processes that can delay procedures and increase staff workload. Selecting disposable devices streamlines clinical operations and improves overall efficiency by minimizing turnaround times between patient uses.
Trends and Future Outlook in Medical Device Usage
The medical device market shows a growing preference for disposable devices due to stringent infection control regulations and the rising incidence of hospital-acquired infections. However, advancements in sterilization technologies and sustainable materials are revitalizing reusable devices by reducing operational costs and environmental impact. Future trends indicate a balanced integration of both device types, driven by innovations in single-use device design and eco-friendly reusable solutions.
Disposable device vs Reusable device Infographic
 productdif.com
productdif.com