Sterile packaging ensures that medical devices remain free from all viable microorganisms by sealing them in a sterilized environment, which is crucial for preserving device safety and efficacy during storage and transportation. Aseptic packaging involves assembling and sealing devices in a contamination-controlled environment to prevent microbial contamination, but unlike sterile packaging, it does not guarantee complete sterility after packaging. Choosing between sterile and aseptic packaging depends on the device's intended use, regulatory requirements, and the level of microbial control necessary to protect patient health.
Table of Comparison
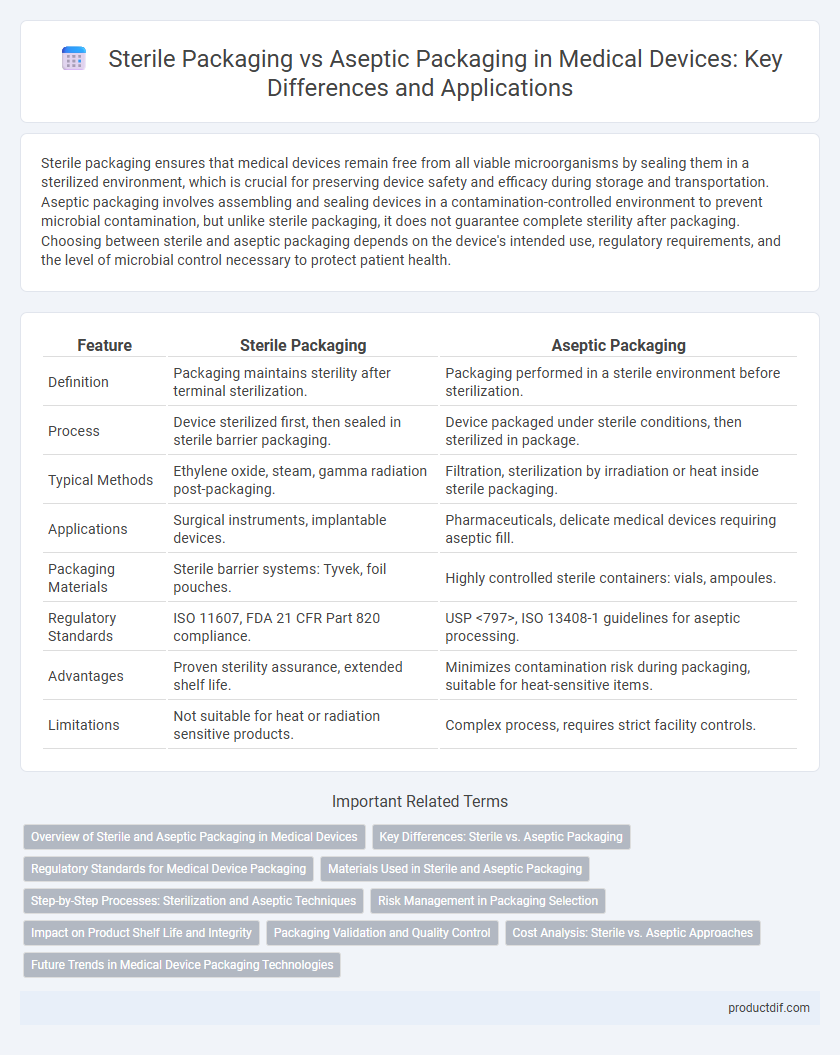
| Feature | Sterile Packaging | Aseptic Packaging |
|---|---|---|
| Definition | Packaging maintains sterility after terminal sterilization. | Packaging performed in a sterile environment before sterilization. |
| Process | Device sterilized first, then sealed in sterile barrier packaging. | Device packaged under sterile conditions, then sterilized in package. |
| Typical Methods | Ethylene oxide, steam, gamma radiation post-packaging. | Filtration, sterilization by irradiation or heat inside sterile packaging. |
| Applications | Surgical instruments, implantable devices. | Pharmaceuticals, delicate medical devices requiring aseptic fill. |
| Packaging Materials | Sterile barrier systems: Tyvek, foil pouches. | Highly controlled sterile containers: vials, ampoules. |
| Regulatory Standards | ISO 11607, FDA 21 CFR Part 820 compliance. | USP <797>, ISO 13408-1 guidelines for aseptic processing. |
| Advantages | Proven sterility assurance, extended shelf life. | Minimizes contamination risk during packaging, suitable for heat-sensitive items. |
| Limitations | Not suitable for heat or radiation sensitive products. | Complex process, requires strict facility controls. |
Overview of Sterile and Aseptic Packaging in Medical Devices
Sterile packaging in medical devices ensures the product is free from all viable microorganisms through terminal sterilization methods such as gamma irradiation, ethylene oxide, or steam sterilization. Aseptic packaging involves assembling and sealing the device in a sterile environment to maintain sterility without terminal sterilization, often used for heat-sensitive or delicate instruments. Both packaging types are critical to patient safety and regulatory compliance, with sterile packaging offering higher assurance of sterility and aseptic packaging enabling flexible handling of sensitive devices.
Key Differences: Sterile vs. Aseptic Packaging
Sterile packaging ensures a complete barrier against microbial contamination by sterilizing the product and package together, often using methods like ethylene oxide or gamma radiation, guaranteeing the packaged medical device remains sterile until opened. Aseptic packaging involves sterilizing the product and packaging separately, then combining them in a sterile environment to prevent contamination, commonly applied in devices sensitive to heat or radiation. Key differences include sterilization timing, contamination risk, and applicability depending on device sensitivity and packaging complexity.
Regulatory Standards for Medical Device Packaging
Sterile packaging for medical devices must comply with ISO 11607, ensuring the sterile barrier system maintains sterility until the point of use, while aseptic packaging adheres to FDA guidelines focusing on contamination control during manufacturing. Regulatory standards for sterile packaging emphasize microbial barrier properties, package integrity, and validation of sterilization processes. In contrast, aseptic packaging requires stringent environmental controls and validated aseptic processing to prevent microbial entry during device assembly and packaging.
Materials Used in Sterile and Aseptic Packaging
Sterile packaging for medical devices commonly utilizes robust materials such as Tyvek, medical-grade polyethylene, and laminated films that ensure barrier protection against microbes and environmental contaminants. Aseptic packaging employs materials like polypropylene, aluminum foil laminates, and breathable membranes designed to maintain sterility by enabling sterilization after packaging. Material selection hinges on compatibility with sterilization methods, mechanical strength, and ability to maintain sterile barriers throughout the product's shelf life.
Step-by-Step Processes: Sterilization and Aseptic Techniques
Sterile packaging involves a defined sterilization process where medical devices are sealed in containers and subjected to methods like steam, ethylene oxide, or gamma radiation to eliminate all microorganisms. Aseptic packaging uses aseptic techniques to assemble and seal devices in a sterile environment, preventing microbial contamination without terminal sterilization. Both processes require rigorous quality control and validation to ensure device sterility and patient safety.
Risk Management in Packaging Selection
Sterile packaging ensures the medical device remains free from viable microorganisms post-packaging, minimizing infection risks critical in surgical and implantable devices. Aseptic packaging involves sterilizing the product and packaging separately before combining them in a sterile environment, which requires stringent environmental controls to prevent contamination. Effective risk management in packaging selection prioritizes sterile packaging for devices with direct patient contact, balancing microbial barrier integrity with regulatory compliance and process validation.
Impact on Product Shelf Life and Integrity
Sterile packaging ensures that medical devices remain free from viable microorganisms by creating an airtight, contamination-resistant barrier, significantly extending product shelf life and maintaining device integrity. Aseptic packaging, while also minimizing microbial contamination through controlled processing environments, relies on maintaining sterility during filling but typically offers shorter shelf life compared to sterile packaging due to potential post-process contamination risks. Optimizing packaging selection based on device sensitivity and shelf life requirements is crucial for maximizing product safety and efficacy.
Packaging Validation and Quality Control
Sterile packaging undergoes rigorous Packaging Validation to ensure barrier integrity and maintain sterility throughout the product's shelf life, utilizing methods such as dye penetration and microbial challenge tests. Aseptic packaging requires meticulous Quality Control processes focusing on maintaining a contaminant-free environment during filling, with validation protocols including environmental monitoring and filter integrity testing. Both packaging types demand comprehensive documentation and compliance with ISO 11607 standards to guarantee patient safety and product efficacy.
Cost Analysis: Sterile vs. Aseptic Approaches
Sterile packaging typically incurs higher costs due to the need for terminal sterilization processes, advanced materials, and stringent validation protocols to ensure product safety. Aseptic packaging demands significant investment in cleanroom infrastructure and controlled environments, which drives up initial capital expenditure but can reduce some operational costs over time. Comparing cost efficiency depends on production scale, product sensitivity, and regulatory requirements, with sterile packaging often favored for single-use devices and aseptic packaging for complex biologics.
Future Trends in Medical Device Packaging Technologies
Future trends in medical device packaging technologies emphasize advancements in sterile packaging that enhance microbial barrier properties through novel multilayer film materials and smart sensor integration for real-time sterility monitoring. Aseptic packaging is evolving with automated closed systems and robotics to minimize contamination risks, while incorporating eco-friendly biopolymers to meet sustainability goals. Integration of IoT and blockchain technologies will provide traceability and quality assurance throughout the device lifecycle, driving innovation in compliance and patient safety.
Sterile packaging vs aseptic packaging Infographic
 productdif.com
productdif.com