Benchtop testing of medical devices for pets provides controlled, repeatable data on safety and functionality before clinical use. Clinical evaluation involves real-world testing on animals, offering insights into performance, safety, and usability under practical conditions. Combining both methods ensures comprehensive assessment for veterinary medical device approval.
Table of Comparison
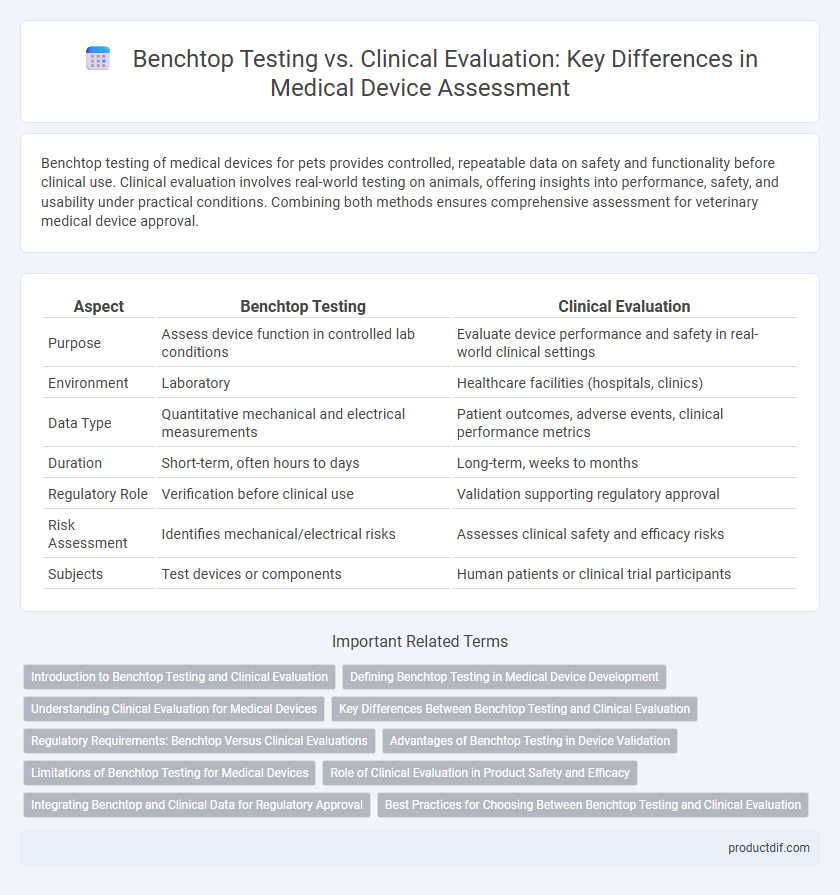
| Aspect | Benchtop Testing | Clinical Evaluation |
|---|---|---|
| Purpose | Assess device function in controlled lab conditions | Evaluate device performance and safety in real-world clinical settings |
| Environment | Laboratory | Healthcare facilities (hospitals, clinics) |
| Data Type | Quantitative mechanical and electrical measurements | Patient outcomes, adverse events, clinical performance metrics |
| Duration | Short-term, often hours to days | Long-term, weeks to months |
| Regulatory Role | Verification before clinical use | Validation supporting regulatory approval |
| Risk Assessment | Identifies mechanical/electrical risks | Assesses clinical safety and efficacy risks |
| Subjects | Test devices or components | Human patients or clinical trial participants |
Introduction to Benchtop Testing and Clinical Evaluation
Benchtop testing involves evaluating medical devices in controlled laboratory settings using standardized protocols to assess performance, safety, and functionality before human use. Clinical evaluation encompasses systematic assessment of clinical data, including clinical trials and real-world evidence, to demonstrate device safety and effectiveness in patient care. Combining benchtop testing and clinical evaluation ensures comprehensive validation of medical devices throughout development and regulatory approval processes.
Defining Benchtop Testing in Medical Device Development
Benchtop testing in medical device development refers to the controlled laboratory evaluation of device components and systems to assess functionality, safety, and performance before clinical use. It involves simulated conditions that replicate physiological environments, enabling engineers to identify design flaws and optimize device parameters without patient risk. This phase is critical for regulatory submissions as it provides essential preclinical data demonstrating device reliability and compliance with industry standards.
Understanding Clinical Evaluation for Medical Devices
Clinical evaluation for medical devices involves a systematic assessment of clinical data to verify safety and performance according to regulatory requirements. Unlike benchtop testing, which focuses on laboratory-based mechanical and functional tests, clinical evaluation integrates real-world patient data, clinical studies, and post-market surveillance. This process ensures comprehensive evidence for device efficacy, risk management, and compliance with standards such as ISO 14155 and MDR 2017/745.
Key Differences Between Benchtop Testing and Clinical Evaluation
Benchtop testing involves controlled laboratory experiments to assess the mechanical and functional performance of medical devices, focusing on reproducibility and precision under simulated conditions. Clinical evaluation collects real-world data on safety, efficacy, and usability through trials involving human subjects, capturing device interactions in actual medical settings. The key differences lie in their environments, data types, and regulatory roles, with benchtop testing providing initial validation and clinical evaluation ensuring compliance with health authorities' requirements for market approval.
Regulatory Requirements: Benchtop Versus Clinical Evaluations
Benchtop testing in medical device development primarily addresses mechanical, electrical, and software performance under controlled laboratory conditions to meet regulatory standards such as ISO 13485 and IEC 60601. Clinical evaluation involves systematic assessment of clinical data to validate device safety and efficacy, fulfilling requirements outlined in regulations like MDR 2017/745 and FDA 21 CFR Part 820. Regulatory submissions rely on both benchtop and clinical evaluations to demonstrate comprehensive compliance with performance and safety criteria mandated by global health authorities.
Advantages of Benchtop Testing in Device Validation
Benchtop testing in medical device validation offers precise control over test variables, enabling reproducible and consistent results critical for early-stage performance assessment. This method allows for rapid identification and correction of design flaws without patient risk, significantly reducing development time and cost. Furthermore, benchtop testing provides quantitative data essential for regulatory submissions, complementing clinical evaluation by verifying the device's mechanical and electrical functions under standardized conditions.
Limitations of Benchtop Testing for Medical Devices
Benchtop testing for medical devices is limited by its inability to fully replicate human physiological conditions, which can result in incomplete assessment of device performance and safety. It often fails to capture complex interactions within the human body, including immunological responses and real-time mechanical stresses experienced during clinical use. These limitations necessitate comprehensive clinical evaluation to ensure accurate validation and regulatory compliance for safe and effective patient outcomes.
Role of Clinical Evaluation in Product Safety and Efficacy
Clinical evaluation plays a critical role in ensuring medical device safety and efficacy by systematically assessing clinical data related to the device's performance in real-world conditions. Unlike benchtop testing, which focuses on mechanical and technical specifications under controlled laboratory settings, clinical evaluation provides evidence of actual patient outcomes, adverse events, and risk-benefit profiles. Regulatory bodies prioritize comprehensive clinical evaluation reports to validate clinical claims and support marketing approval, thereby safeguarding patient health and treatment effectiveness.
Integrating Benchtop and Clinical Data for Regulatory Approval
Integrating benchtop testing with clinical evaluation provides a comprehensive data set essential for regulatory approval of medical devices. Benchtop testing offers controlled, reproducible mechanical and safety performance data, while clinical evaluation delivers real-world evidence of device effectiveness and patient outcomes. Combining these data sets enhances regulatory submissions by demonstrating both device reliability and clinical benefits, facilitating approval by agencies such as the FDA and EMA.
Best Practices for Choosing Between Benchtop Testing and Clinical Evaluation
Benchtop testing offers controlled, reproducible conditions for initial assessment of medical device functionality, safety, and durability, making it ideal for early-stage prototype validation and iterative design improvements. Clinical evaluation provides essential real-world evidence on device performance, safety, and user experience with patient populations, crucial for regulatory submissions and post-market surveillance. Best practices recommend integrating both methods, starting with rigorous benchtop testing to identify technical issues, followed by targeted clinical evaluation to confirm efficacy and safety in actual clinical settings.
Benchtop testing vs Clinical evaluation Infographic
 productdif.com
productdif.com