Risk Management (ISO 14971) systematically identifies, evaluates, and mitigates potential hazards associated with medical devices to ensure patient safety throughout the product lifecycle. Quality Management (ISO 13485) establishes a comprehensive framework for consistent product design, manufacturing, and post-market activities, emphasizing regulatory compliance and customer satisfaction. Integrating both standards enhances device reliability by aligning safety risk controls with quality processes and regulatory requirements.
Table of Comparison
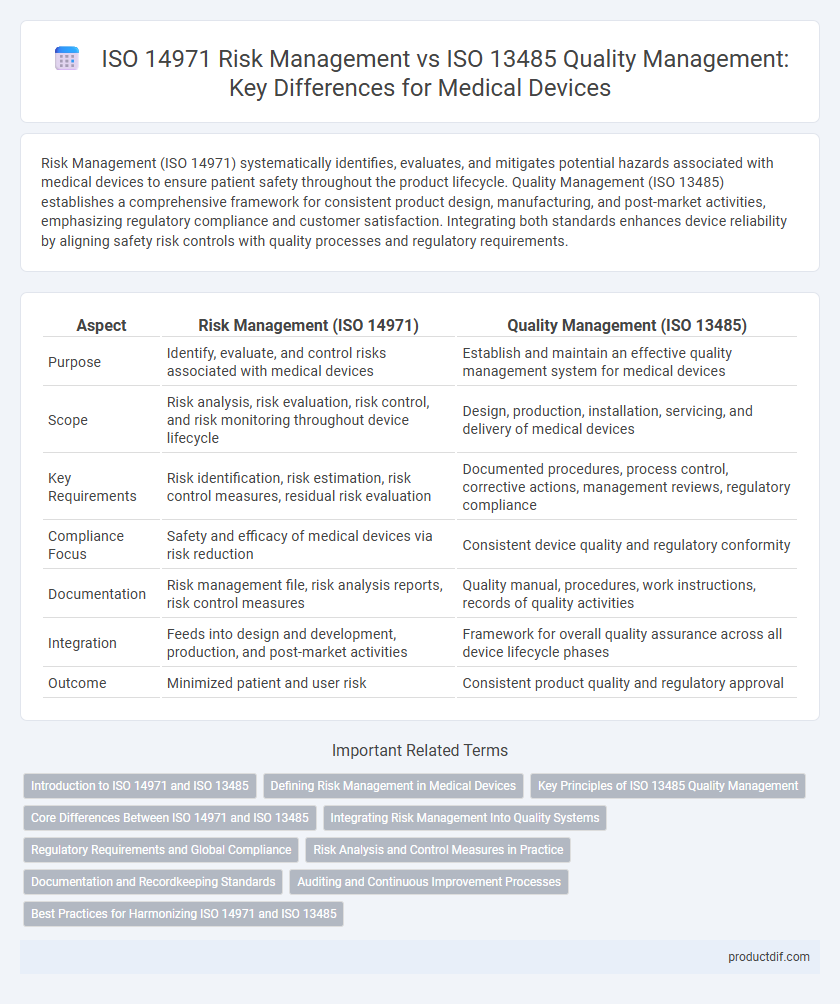
| Aspect | Risk Management (ISO 14971) | Quality Management (ISO 13485) |
|---|---|---|
| Purpose | Identify, evaluate, and control risks associated with medical devices | Establish and maintain an effective quality management system for medical devices |
| Scope | Risk analysis, risk evaluation, risk control, and risk monitoring throughout device lifecycle | Design, production, installation, servicing, and delivery of medical devices |
| Key Requirements | Risk identification, risk estimation, risk control measures, residual risk evaluation | Documented procedures, process control, corrective actions, management reviews, regulatory compliance |
| Compliance Focus | Safety and efficacy of medical devices via risk reduction | Consistent device quality and regulatory conformity |
| Documentation | Risk management file, risk analysis reports, risk control measures | Quality manual, procedures, work instructions, records of quality activities |
| Integration | Feeds into design and development, production, and post-market activities | Framework for overall quality assurance across all device lifecycle phases |
| Outcome | Minimized patient and user risk | Consistent product quality and regulatory approval |
Introduction to ISO 14971 and ISO 13485
ISO 14971 provides a structured framework for identifying, assessing, and controlling risks associated with medical devices throughout their lifecycle to ensure patient safety. ISO 13485 defines comprehensive quality management system requirements specific to the medical device industry, emphasizing regulatory compliance and consistent product quality. Integration of ISO 14971 risk management processes within the ISO 13485 quality system enhances overall device safety and effectiveness.
Defining Risk Management in Medical Devices
Risk Management in medical devices, as defined by ISO 14971, involves the systematic identification, evaluation, control, and monitoring of risks associated with medical device use, ensuring patient safety and compliance. It includes hazard analysis, risk estimation, risk evaluation, and risk control measures tailored to device-specific hazards throughout the product lifecycle. This process integrates with Quality Management System requirements outlined in ISO 13485 but focuses specifically on minimizing potential harm from device-related risks.
Key Principles of ISO 13485 Quality Management
ISO 13485 Quality Management emphasizes consistent design, production, and post-production processes to ensure medical device safety and regulatory compliance. Key principles include establishing a robust documentation system, implementing effective corrective and preventive actions (CAPA), and maintaining stringent supplier controls. This standard prioritizes risk-based decision making to enhance product quality throughout the entire lifecycle of medical devices.
Core Differences Between ISO 14971 and ISO 13485
ISO 14971 focuses specifically on risk management processes for medical devices, emphasizing hazard identification, risk analysis, evaluation, control, and monitoring throughout the product lifecycle. ISO 13485 establishes a comprehensive quality management system framework, targeting consistent design, production, and regulatory compliance to ensure device safety and effectiveness. The core difference lies in ISO 14971's detailed approach to risk control strategies versus ISO 13485's broader scope on quality system requirements and documentation.
Integrating Risk Management Into Quality Systems
Integrating Risk Management (ISO 14971) into Quality Management Systems (ISO 13485) ensures a comprehensive approach to medical device safety and regulatory compliance by embedding risk assessment, control, and monitoring within quality processes. ISO 14971 provides a systematic framework for identifying and mitigating risks, while ISO 13485 establishes requirements for a robust quality management system emphasizing continuous improvement. Aligning these standards enhances product reliability, minimizes hazards, and supports regulatory submissions by maintaining documented evidence of risk controls throughout the device lifecycle.
Regulatory Requirements and Global Compliance
Risk Management under ISO 14971 mandates identification, evaluation, and control of hazards throughout the medical device lifecycle to ensure patient safety, aligning with stringent regulatory requirements from agencies like FDA and EMA. Quality Management according to ISO 13485 establishes a comprehensive framework for consistent product quality and regulatory compliance, encompassing design, production, and post-market activities globally. Together, adherence to these standards facilitates robust regulatory submissions, reduces liability, and supports market access in diverse international markets.
Risk Analysis and Control Measures in Practice
Risk management under ISO 14971 involves systematic risk analysis, evaluation, and implementation of control measures to ensure medical device safety throughout its lifecycle. Quality management per ISO 13485 integrates these risk control measures within manufacturing processes to maintain product consistency and compliance with regulatory requirements. Practical application requires continuous monitoring and verification of risk controls to minimize hazards and uphold device efficacy and patient safety.
Documentation and Recordkeeping Standards
Risk Management under ISO 14971 mandates comprehensive documentation of risk analysis, evaluation, control measures, and residual risks to ensure traceability and compliance with safety standards. Quality Management following ISO 13485 emphasizes systematic recordkeeping of manufacturing processes, quality controls, and corrective actions to maintain product consistency and regulatory adherence. Both standards require meticulous documentation practices but ISO 14971 focuses on risk control lifecycle records while ISO 13485 centers on quality system records for medical device certification.
Auditing and Continuous Improvement Processes
Risk Management under ISO 14971 focuses on identifying, evaluating, and mitigating risks associated with medical devices throughout their lifecycle, ensuring device safety and compliance. Quality Management according to ISO 13485 emphasizes establishing and maintaining effective processes for product quality, including rigorous auditing and systematic continuous improvement to meet regulatory requirements. Auditing within ISO 13485 verifies the effectiveness of both risk control measures from ISO 14971 and overall process performance, driving continuous improvement and fostering a culture of quality and safety in medical device manufacturing.
Best Practices for Harmonizing ISO 14971 and ISO 13485
Harmonizing ISO 14971 and ISO 13485 involves integrating risk management processes directly into the quality management system to ensure comprehensive compliance and enhanced product safety. Best practices include aligning risk assessment activities with quality objectives, documenting risk controls within design and production procedures, and establishing continuous monitoring to track risk effectiveness throughout the product lifecycle. This approach supports seamless regulatory adherence and drives proactive risk mitigation in medical device manufacturing.
Risk Management (ISO 14971) vs Quality Management (ISO 13485) Infographic
 productdif.com
productdif.com