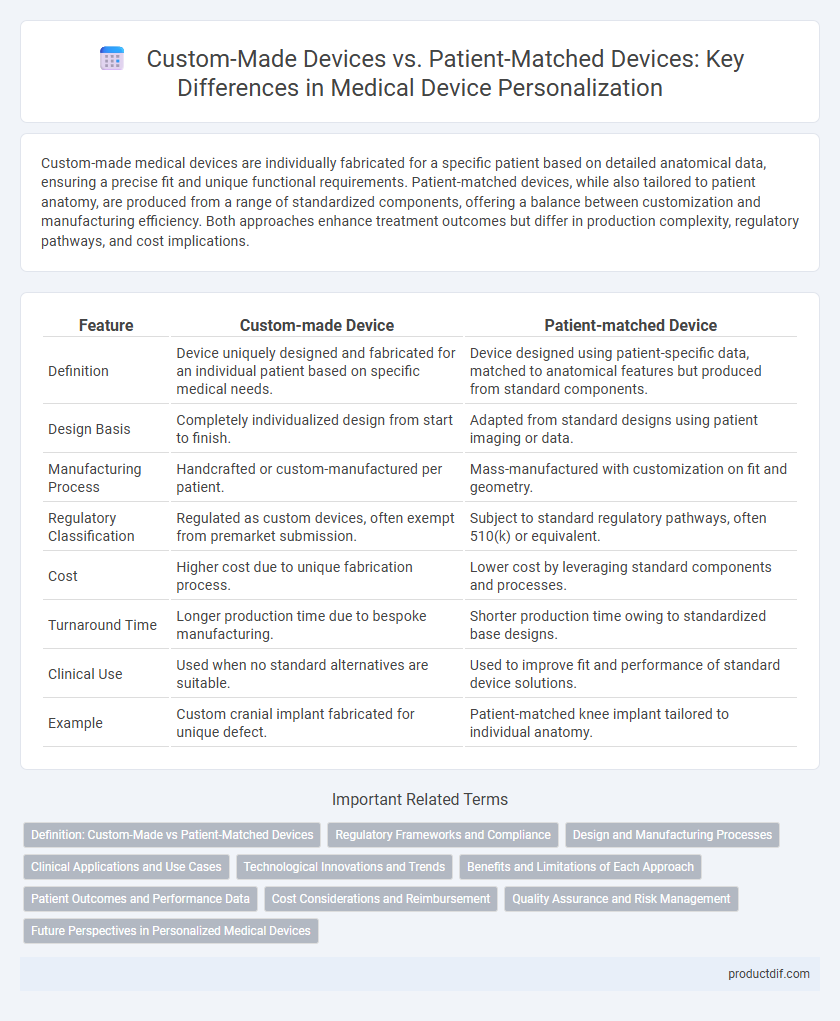
Custom-made medical devices are individually fabricated for a specific patient based on detailed anatomical data, ensuring a precise fit and unique functional requirements. Patient-matched devices, while also tailored to patient anatomy, are produced from a range of standardized components, offering a balance between customization and manufacturing efficiency. Both approaches enhance treatment outcomes but differ in production complexity, regulatory pathways, and cost implications.
Table of Comparison
| Feature | Custom-made Device | Patient-matched Device |
|---|---|---|
| Definition | Device uniquely designed and fabricated for an individual patient based on specific medical needs. | Device designed using patient-specific data, matched to anatomical features but produced from standard components. |
| Design Basis | Completely individualized design from start to finish. | Adapted from standard designs using patient imaging or data. |
| Manufacturing Process | Handcrafted or custom-manufactured per patient. | Mass-manufactured with customization on fit and geometry. |
| Regulatory Classification | Regulated as custom devices, often exempt from premarket submission. | Subject to standard regulatory pathways, often 510(k) or equivalent. |
| Cost | Higher cost due to unique fabrication process. | Lower cost by leveraging standard components and processes. |
| Turnaround Time | Longer production time due to bespoke manufacturing. | Shorter production time owing to standardized base designs. |
| Clinical Use | Used when no standard alternatives are suitable. | Used to improve fit and performance of standard device solutions. |
| Example | Custom cranial implant fabricated for unique defect. | Patient-matched knee implant tailored to individual anatomy. |
Definition: Custom-Made vs Patient-Matched Devices
Custom-made medical devices are individually manufactured according to a specific prescription for a single patient, tailored to meet unique anatomical or clinical needs. Patient-matched devices rely on imaging or digitized data to produce components that align with the patient's anatomy but use standardized manufacturing processes. Understanding the distinction aids in regulatory compliance and informs clinical decision-making based on customization level and production methodology.
Regulatory Frameworks and Compliance
Custom-made medical devices are typically exempt from certain regulatory requirements under frameworks like the EU Medical Device Regulation (MDR), which grants manufacturers flexibility when producing devices tailored to individual patient needs without mass production. Patient-matched devices, however, must comply with full conformity assessment procedures, including strict quality management system requirements and clinical evaluation, ensuring consistency and safety despite customization. Regulatory compliance for both device types necessitates thorough documentation but varies significantly in terms of pre-market approval processes and post-market surveillance obligations.
Design and Manufacturing Processes
Custom-made devices are individually designed and fabricated for a unique patient based on specific medical prescriptions, involving a highly personalized design process and manual craftsmanship. Patient-matched devices are produced using standardized design templates tailored through advanced digital imaging and computer-aided manufacturing to fit the patient's anatomy more efficiently. Both approaches leverage 3D printing and CAD/CAM technologies, but custom-made devices emphasize bespoke fabrication while patient-matched devices optimize scalability and consistency in production.
Clinical Applications and Use Cases
Custom-made medical devices are designed and manufactured for a specific patient based on a unique prescription, often used in cases requiring highly individualized implants such as cranial plates or orthopedic prosthetics. Patient-matched devices leverage standardized templates adjusted to a patient's anatomy through imaging data, commonly applied in orthopedic surgery and dental restorations for improved fit and function. Both approaches enhance clinical outcomes by addressing patient-specific anatomical variations, with custom-made devices offering greater personalization in complex cases and patient-matched devices providing scalable solutions for routine procedures.
Technological Innovations and Trends
Custom-made medical devices are individually designed and fabricated to meet a specific patient's unique anatomy, leveraging advanced 3D printing and CAD technologies for precise customization. Patient-matched devices utilize pre-existing design templates adapted using imaging data to fit a patient's anatomy, benefiting from AI-driven modeling and rapid prototyping innovations. Emerging trends emphasize increased integration of machine learning algorithms and biocompatible materials to enhance device performance and reduce production timelines.
Benefits and Limitations of Each Approach
Custom-made medical devices offer precise anatomical conformity and tailored functionality, enhancing treatment outcomes for unique patient needs but often involve longer production times and higher costs. Patient-matched devices leverage advanced imaging and design software to provide near-custom fit with faster manufacturing and scalability, though may lack the exact personalization of custom-made solutions. Both approaches improve patient care by addressing individual anatomical variations, yet trade-offs exist between customization depth, production efficiency, and cost-effectiveness.
Patient Outcomes and Performance Data
Custom-made medical devices are individually designed and fabricated for a single patient, often lacking extensive performance data and standardized validation, which may lead to variability in patient outcomes. Patient-matched devices utilize pre-existing, validated designs tailored to patient-specific anatomical data, offering improved predictability and consistency in clinical performance. Studies indicate patient-matched devices generally achieve better functional outcomes and lower complication rates due to rigorous testing and optimized design parameters.
Cost Considerations and Reimbursement
Custom-made medical devices often incur higher upfront manufacturing costs due to individualized design and specialized materials, affecting overall budget planning. Patient-matched devices, designed using adaptable digital workflows, can reduce development expenses and improve scalability, thereby influencing reimbursement strategies favorably. Insurance coverage and reimbursement policies typically vary, with patient-matched devices more likely to receive streamlined approval due to standardized production processes and documented clinical efficacy.
Quality Assurance and Risk Management
Custom-made devices require stringent Quality Assurance protocols tailored to individual specifications, ensuring compliance with regulatory standards such as ISO 13485 and MDR Annex I. Patient-matched devices benefit from standardized design controls and risk management frameworks, including ISO 14971 risk analysis, which reduce variability and enhance predictability of clinical outcomes. Effective risk management in both device types involves comprehensive documentation, traceability, and validation processes to mitigate adverse events and ensure patient safety.
Future Perspectives in Personalized Medical Devices
Future perspectives in personalized medical devices emphasize advancements in patient-matched devices that leverage imaging and CAD/CAM technologies for precise anatomical fit, enhancing clinical outcomes and reducing production times compared to traditional custom-made devices. Integration of artificial intelligence and machine learning algorithms is expected to optimize design processes, enabling dynamic adaptation to patient-specific pathophysiology. Adoption of biocompatible and smart materials will further support real-time monitoring and therapeutic responsiveness, revolutionizing personalized healthcare delivery.
Custom-made device vs Patient-matched device Infographic
 productdif.com
productdif.com