Clinical validation ensures that a medical device accurately measures the intended clinical condition by demonstrating its effectiveness and safety in real-world patient settings. Analytical validation focuses on the device's technical performance, verifying precision, accuracy, sensitivity, and specificity under controlled laboratory conditions. Both validations are essential to confirm that the device reliably supports clinical decisions and meets regulatory standards.
Table of Comparison
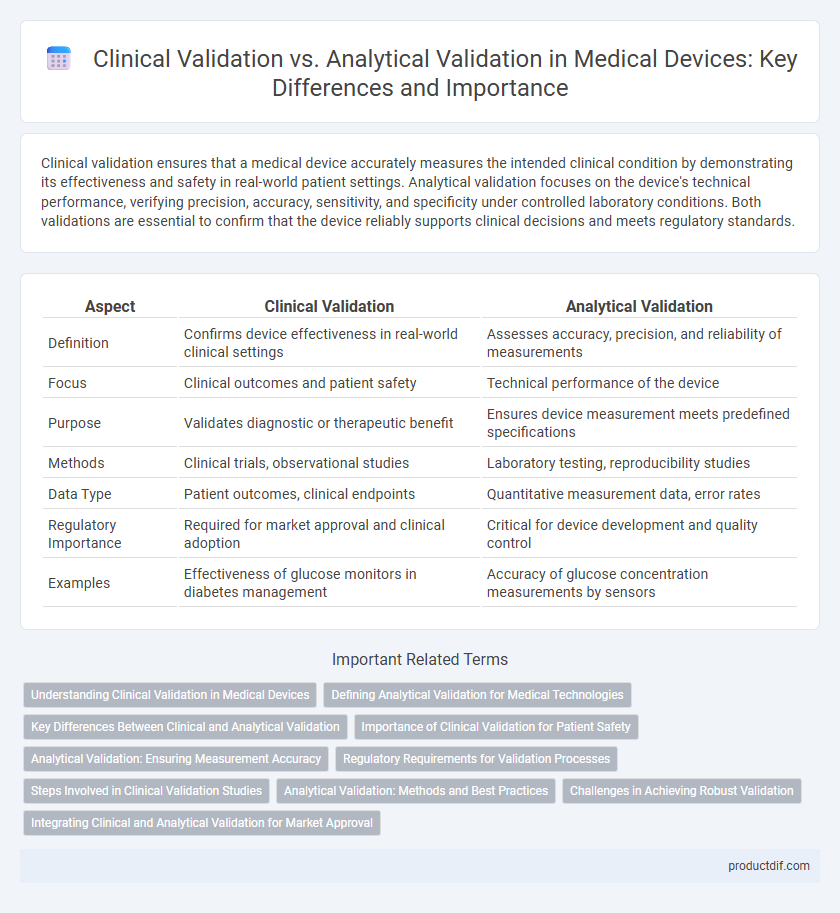
| Aspect | Clinical Validation | Analytical Validation |
|---|---|---|
| Definition | Confirms device effectiveness in real-world clinical settings | Assesses accuracy, precision, and reliability of measurements |
| Focus | Clinical outcomes and patient safety | Technical performance of the device |
| Purpose | Validates diagnostic or therapeutic benefit | Ensures device measurement meets predefined specifications |
| Methods | Clinical trials, observational studies | Laboratory testing, reproducibility studies |
| Data Type | Patient outcomes, clinical endpoints | Quantitative measurement data, error rates |
| Regulatory Importance | Required for market approval and clinical adoption | Critical for device development and quality control |
| Examples | Effectiveness of glucose monitors in diabetes management | Accuracy of glucose concentration measurements by sensors |
Understanding Clinical Validation in Medical Devices
Clinical validation in medical devices ensures that the device performs effectively and safely in real-world patient settings by demonstrating clinical benefits and outcomes. It involves evaluating the device's impact on patient health through rigorous clinical trials and studies, differentiating it from analytical validation which focuses on the device's technical accuracy and reliability in controlled environments. Understanding clinical validation is critical for regulatory approval and market adoption, as it confirms the device's practical efficacy and safety in target populations.
Defining Analytical Validation for Medical Technologies
Analytical validation for medical technologies involves verifying that a device accurately and reliably measures the intended analyte or parameter under predefined conditions. This process encompasses assessing precision, accuracy, sensitivity, specificity, linearity, and reproducibility to ensure measurement consistency and reliability. Unlike clinical validation, which evaluates clinical performance and patient outcomes, analytical validation focuses on the technical and functional performance of the device in controlled settings.
Key Differences Between Clinical and Analytical Validation
Clinical validation assesses a medical device's ability to accurately detect or predict a clinical condition in real-world patient populations, emphasizing diagnostic accuracy, sensitivity, and specificity. Analytical validation focuses on the device's technical performance, including precision, accuracy, limit of detection, reproducibility, and robustness in controlled laboratory settings. Key differences lie in the validation environment, objectives, and performance metrics, with clinical validation ensuring clinical relevance and analytical validation confirming technical reliability.
Importance of Clinical Validation for Patient Safety
Clinical validation ensures medical devices perform accurately and safely in real-world patient settings, directly impacting patient outcomes and reducing risks of misdiagnosis or harm. Analytical validation confirms the device's technical accuracy and precision but does not guarantee clinical effectiveness or safety in diverse patient populations. Emphasizing clinical validation is crucial for regulatory approval and maintaining high standards of patient safety in medical device deployment.
Analytical Validation: Ensuring Measurement Accuracy
Analytical validation in medical device development ensures precise measurement accuracy by systematically evaluating the device's sensitivity, specificity, linearity, and reproducibility. This process confirms that the device consistently produces reliable and quantifiable results under defined conditions, minimizing measurement errors. Accurate analytical validation is critical for device performance, enabling regulatory approval and consistent clinical utility.
Regulatory Requirements for Validation Processes
Clinical validation demonstrates a medical device's ability to provide accurate and reliable patient outcomes in real-world settings, meeting regulatory requirements set by agencies like the FDA and EMA. Analytical validation focuses on verifying the device's technical performance, such as accuracy, precision, sensitivity, and specificity, according to standards outlined in ISO 13485 and IEC 62304. Regulatory guidelines demand comprehensive documentation for both validation processes to ensure device safety, efficacy, and compliance before market approval.
Steps Involved in Clinical Validation Studies
Clinical validation studies in medical devices primarily involve patient recruitment based on strict inclusion and exclusion criteria, followed by sample collection and testing using the device under investigation to evaluate clinical accuracy and diagnostic performance. Data analysis includes statistical assessments such as sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) to determine clinical utility. These steps ensure the device's effectiveness in real-world clinical settings, distinguishing clinical validation from analytical validation, which focuses on technical performance metrics like precision and limit of detection.
Analytical Validation: Methods and Best Practices
Analytical validation in medical devices ensures the accuracy, precision, sensitivity, and specificity of diagnostic algorithms or instruments through rigorous testing of analytical performance. Methods include assessing linearity, limit of detection, reproducibility, and calibration using standardized reference materials and protocols. Best practices involve implementing robust quality controls, continuous monitoring of assay performance, and adherence to regulatory guidelines such as FDA and ISO 13485 standards.
Challenges in Achieving Robust Validation
Clinical validation of medical devices faces challenges such as patient variability, complex biological interactions, and ethical considerations that affect real-world performance assessment. Analytical validation struggles with ensuring accuracy, precision, and reproducibility amid diverse sample matrices and potential interference factors. Bridging these validations requires integrating robust study design, rigorous statistical analysis, and comprehensive data management to ensure reliable and clinically meaningful outcomes.
Integrating Clinical and Analytical Validation for Market Approval
Clinical validation ensures a medical device's safety and effectiveness in real-world patient settings by demonstrating its clinical performance and impact on health outcomes. Analytical validation verifies the device's technical accuracy, precision, and reliability through rigorous laboratory testing and standardized protocols. Integrating clinical and analytical validation accelerates market approval by providing comprehensive evidence that satisfies regulatory requirements for both functional integrity and clinical relevance.
Clinical validation vs Analytical validation Infographic
 productdif.com
productdif.com