Unique Device Identification (UDI) provides a standardized system for tracking medical devices throughout their lifecycle, enhancing patient safety and regulatory compliance. Unlike a serial number that uniquely identifies a single device, a UDI includes both a device identifier and a production identifier, offering comprehensive traceability. Implementing UDI enables healthcare providers and manufacturers to efficiently manage recalls, monitor device performance, and improve inventory control.
Table of Comparison
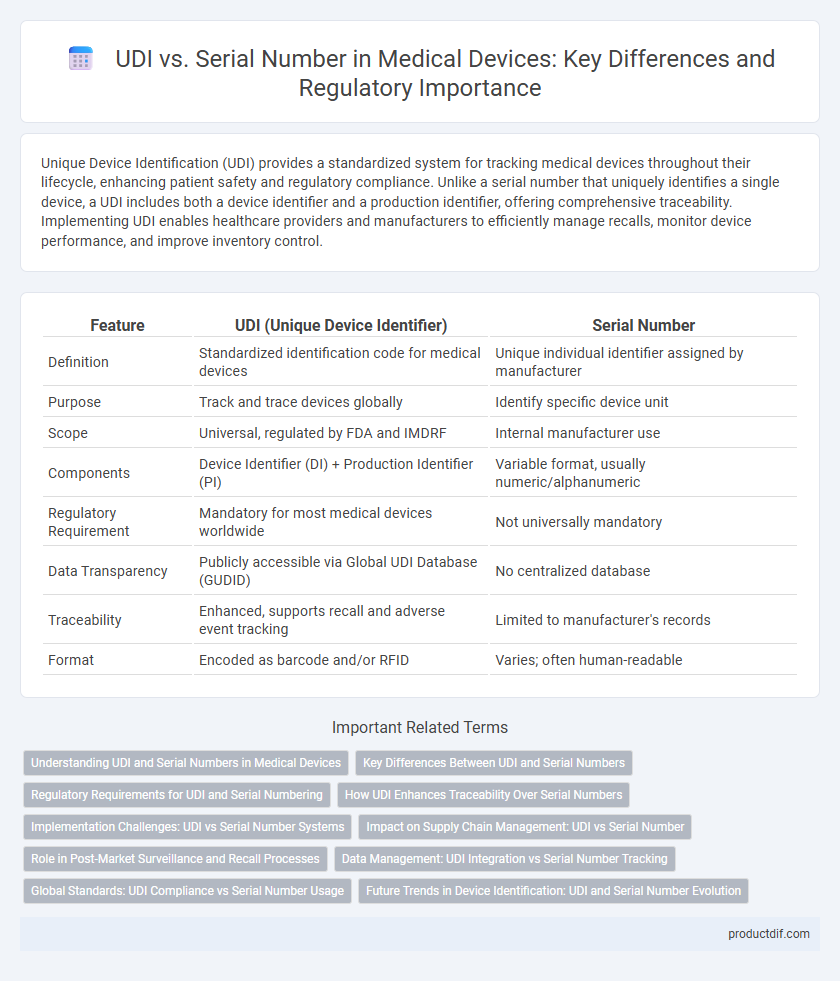
| Feature | UDI (Unique Device Identifier) | Serial Number |
|---|---|---|
| Definition | Standardized identification code for medical devices | Unique individual identifier assigned by manufacturer |
| Purpose | Track and trace devices globally | Identify specific device unit |
| Scope | Universal, regulated by FDA and IMDRF | Internal manufacturer use |
| Components | Device Identifier (DI) + Production Identifier (PI) | Variable format, usually numeric/alphanumeric |
| Regulatory Requirement | Mandatory for most medical devices worldwide | Not universally mandatory |
| Data Transparency | Publicly accessible via Global UDI Database (GUDID) | No centralized database |
| Traceability | Enhanced, supports recall and adverse event tracking | Limited to manufacturer's records |
| Format | Encoded as barcode and/or RFID | Varies; often human-readable |
Understanding UDI and Serial Numbers in Medical Devices
Unique Device Identification (UDI) is a standardized system mandated by regulatory authorities to improve the traceability and safety of medical devices throughout their lifecycle. Unlike serial numbers, which uniquely identify individual devices within a manufacturer's production batch, UDIs consist of a device identifier (DI) and a production identifier (PI), providing detailed information about the device model, manufacturing date, and expiration. Implementing UDI enhances inventory accuracy, supports adverse event reporting, and facilitates recalls by linking clinical data seamlessly with specific devices.
Key Differences Between UDI and Serial Numbers
Unique Device Identifier (UDI) provides a standardized code that includes device-specific information such as manufacturer, model, and production batch, enabling global tracking and regulatory compliance. Serial numbers serve as unique codes assigned to individual medical devices, allowing for precise identification and traceability within a manufacturer's inventory system. While UDI emphasizes regulatory reporting and supply chain transparency, serial numbers focus on detailed device-level tracking and warranty management.
Regulatory Requirements for UDI and Serial Numbering
Unique Device Identification (UDI) is mandatory under FDA regulations for medical devices to enhance traceability and patient safety, requiring compliance with specific formatting, submission to the Global Unique Device Identification Database (GUDID), and adherence to labeling standards. Serial numbers serve as device-specific identifiers, enabling manufacturers to track individual units for quality control and recall management, but they do not fulfill regulatory obligations for public device identification like the UDI. Regulatory frameworks prioritize UDI for standardized identification and risk management, while serial numbering supports internal traceability and post-market surveillance.
How UDI Enhances Traceability Over Serial Numbers
Unique Device Identification (UDI) enhances traceability by providing standardized, globally recognized codes that link medical devices to key regulatory and safety information, unlike traditional serial numbers which are often device-specific and lack uniformity. UDIs enable more efficient tracking across supply chains, healthcare facilities, and regulatory databases, facilitating faster response during recalls or adverse events. This system improves patient safety and device management by integrating detailed product data, manufacturing details, and expiration information in a consistent format.
Implementation Challenges: UDI vs Serial Number Systems
Implementing Unique Device Identification (UDI) systems poses greater complexity compared to traditional serial number tracking due to regulatory requirements from bodies like the FDA and the need for standardized formats across global supply chains. UDI demands integration with electronic health records and post-market surveillance databases, increasing the technical and operational burden on manufacturers and healthcare providers. Serial number systems, while simpler, lack the comprehensive traceability and interoperability that UDI systems provide, potentially limiting their effectiveness in product recalls and adverse event investigations.
Impact on Supply Chain Management: UDI vs Serial Number
Unique Device Identification (UDI) enhances supply chain management by providing a standardized system for tracking medical devices through global distribution channels, improving traceability and recall efficiency. Unlike traditional serial numbers, which only identify individual units without industry-wide standardization, UDIs facilitate regulatory compliance and interoperability across diverse healthcare systems. Implementing UDIs reduces inventory errors, streamlines logistics, and supports real-time data sharing among manufacturers, distributors, and healthcare providers.
Role in Post-Market Surveillance and Recall Processes
The Unique Device Identifier (UDI) system enhances post-market surveillance by enabling precise tracking of medical devices throughout their lifecycle, facilitating efficient identification during adverse event reporting and recall processes. Unlike traditional serial numbers, UDIs provide standardized data that regulatory bodies and healthcare providers use to rapidly detect device-related issues and implement corrective actions. This improves patient safety by ensuring swift, accurate recall management and comprehensive monitoring of device performance in real-world use.
Data Management: UDI Integration vs Serial Number Tracking
UDI integration streamlines data management by providing a standardized global identification system that enhances traceability and regulatory compliance across medical devices. Serial number tracking offers unique device-level identification but lacks the universal consistency and interoperability present in UDI systems. Combining UDI with serial number tracking optimizes data accuracy, device lifecycle monitoring, and recall efficiency in medical device management.
Global Standards: UDI Compliance vs Serial Number Usage
Unique Device Identification (UDI) systems comply with global regulatory standards such as those set by the FDA and EU MDR, ensuring traceability and enhancing patient safety through standardized labeling. Serial numbers, while useful for inventory and device-specific tracking, lack the comprehensive regulatory framework and universal applicability that UDI mandates provide. Adoption of UDI supports interoperability across international healthcare systems, facilitating recalls, adverse event reporting, and post-market surveillance more effectively than traditional serial number usage.
Future Trends in Device Identification: UDI and Serial Number Evolution
Future trends in medical device identification emphasize the integration of Unique Device Identification (UDI) with advanced serialization technologies to enhance traceability and patient safety. Emerging innovations include blockchain-based UDI registries and AI-powered data analytics to streamline regulatory compliance and post-market surveillance. Industry adoption of IoT-enabled smart devices will further evolve serial number usage, providing real-time monitoring and predictive maintenance insights.
UDI vs Serial Number Infographic
 productdif.com
productdif.com