ISO 13485 certification ensures that a medical device manufacturer maintains a quality management system tailored to regulatory requirements, promoting consistent product safety and effectiveness. ISO 14971 compliance focuses on the systematic application of risk management throughout the device lifecycle, identifying, evaluating, and controlling potential hazards to minimize patient and user risk. Together, these standards support regulatory approval and market acceptance by emphasizing quality assurance and risk mitigation.
Table of Comparison
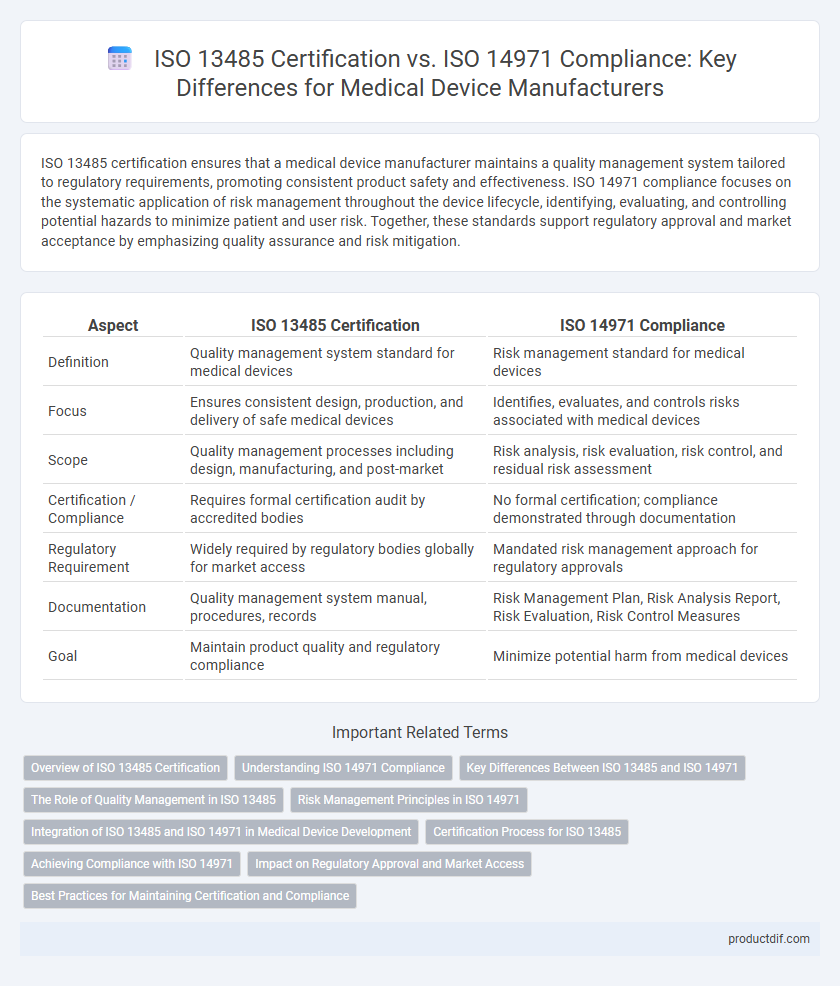
| Aspect | ISO 13485 Certification | ISO 14971 Compliance |
|---|---|---|
| Definition | Quality management system standard for medical devices | Risk management standard for medical devices |
| Focus | Ensures consistent design, production, and delivery of safe medical devices | Identifies, evaluates, and controls risks associated with medical devices |
| Scope | Quality management processes including design, manufacturing, and post-market | Risk analysis, risk evaluation, risk control, and residual risk assessment |
| Certification / Compliance | Requires formal certification audit by accredited bodies | No formal certification; compliance demonstrated through documentation |
| Regulatory Requirement | Widely required by regulatory bodies globally for market access | Mandated risk management approach for regulatory approvals |
| Documentation | Quality management system manual, procedures, records | Risk Management Plan, Risk Analysis Report, Risk Evaluation, Risk Control Measures |
| Goal | Maintain product quality and regulatory compliance | Minimize potential harm from medical devices |
Overview of ISO 13485 Certification
ISO 13485 certification demonstrates a medical device manufacturer's adherence to a comprehensive quality management system tailored to regulatory requirements for medical devices. This certification ensures consistent design, development, production, and delivery of safe and effective medical devices by emphasizing risk management, process control, and regulatory compliance. It differs from ISO 14971, which specifically focuses on risk management processes throughout the product lifecycle rather than the broader quality management framework.
Understanding ISO 14971 Compliance
ISO 14971 compliance centers on risk management for medical devices, detailing a systematic process to identify, evaluate, control, and monitor risks associated with device use throughout its lifecycle. Unlike ISO 13485, which specifies requirements for a quality management system, ISO 14971 emphasizes risk assessment and mitigation to ensure patient safety and regulatory adherence. Mastery of ISO 14971 involves integrating risk management principles into design, production, and post-market activities to meet global regulatory expectations.
Key Differences Between ISO 13485 and ISO 14971
ISO 13485 certification focuses on establishing a comprehensive quality management system specific to medical device manufacturing, ensuring consistent product safety and regulatory compliance. In contrast, ISO 14971 compliance centers on risk management processes for identifying, evaluating, and controlling risks associated with medical devices throughout their lifecycle. While ISO 13485 mandates procedural and documentation controls, ISO 14971 emphasizes systematic risk analysis, risk control measures, and risk-benefit assessments to minimize patient harm.
The Role of Quality Management in ISO 13485
ISO 13485 certification emphasizes the implementation of a comprehensive quality management system (QMS) tailored specifically for medical devices, ensuring consistent product safety and regulatory compliance. It requires documented processes for risk management, supplier control, and corrective actions to maintain device quality throughout the product lifecycle. While ISO 14971 focuses on risk management, ISO 13485 integrates these principles within a broader QMS framework that drives continuous improvement and customer satisfaction.
Risk Management Principles in ISO 14971
ISO 13485 certification establishes a comprehensive quality management system for medical device manufacturers, ensuring product safety and regulatory compliance. ISO 14971 focuses specifically on risk management principles, guiding the identification, evaluation, control, and monitoring of risks throughout the medical device lifecycle. Emphasizing proactive risk assessment, ISO 14971 compliance integrates risk controls that minimize potential hazards while supporting product effectiveness and patient safety.
Integration of ISO 13485 and ISO 14971 in Medical Device Development
ISO 13485 certification ensures a comprehensive quality management system for medical device manufacturers, emphasizing consistent product design and production control. ISO 14971 compliance focuses on systematic risk management throughout the medical device lifecycle, identifying hazards and mitigating risks to ensure patient safety. Integrating ISO 13485 and ISO 14971 streamlines risk-based quality management, enhancing regulatory compliance and accelerating medical device development while maintaining rigorous safety standards.
Certification Process for ISO 13485
ISO 13485 certification requires a comprehensive auditing process that evaluates a medical device manufacturer's quality management system for regulatory compliance and operational effectiveness. The certification process involves documentation review, on-site audits, and corrective action verification to ensure adherence to processes that consistently meet customer and regulatory requirements. In contrast, ISO 14971 compliance focuses on risk management practices and does not require formal certification, but rather documented implementation of risk analysis and mitigation throughout the product lifecycle.
Achieving Compliance with ISO 14971
Achieving compliance with ISO 14971 requires a comprehensive risk management process tailored to medical devices, emphasizing the identification, evaluation, control, and monitoring of potential hazards throughout the product lifecycle. Unlike ISO 13485, which standardizes quality management systems for medical device manufacturing, ISO 14971 specifically targets risk management principles to ensure patient safety and regulatory adherence. Effective implementation of ISO 14971 involves integrating risk management measures into design controls, production processes, and post-market surveillance to maintain continual compliance and mitigate adverse effects.
Impact on Regulatory Approval and Market Access
ISO 13485 certification demonstrates a medical device manufacturer's quality management system meets regulatory requirements, facilitating smoother regulatory approval processes and broader market access globally. ISO 14971 compliance ensures effective risk management throughout the product lifecycle, directly impacting device safety and regulatory submissions by addressing potential hazards systematically. Together, adherence to ISO 13485 and ISO 14971 significantly enhances regulatory authority confidence, accelerating market entry and expanding international opportunities.
Best Practices for Maintaining Certification and Compliance
Implementing a robust Quality Management System (QMS) aligned with ISO 13485 ensures medical device manufacturers consistently meet regulatory requirements and deliver safe products. Regular risk management activities based on ISO 14971, including hazard analysis and risk evaluation, are critical for ongoing compliance and proactive identification of potential device failures. Continuous internal audits, employee training, and documentation updates are best practices that support both certification maintenance and effective risk control.
ISO 13485 certification vs ISO 14971 compliance Infographic
 productdif.com
productdif.com