Post-market surveillance continuously monitors the safety and performance of a medical device throughout its lifecycle, collecting real-world data to identify potential risks and improve product quality. Vigilance reporting specifically involves the mandatory communication of serious adverse events or incidents to regulatory authorities to ensure timely corrective actions. Effective integration of both processes enhances patient safety and regulatory compliance by enabling proactive risk management and swift incident response.
Table of Comparison
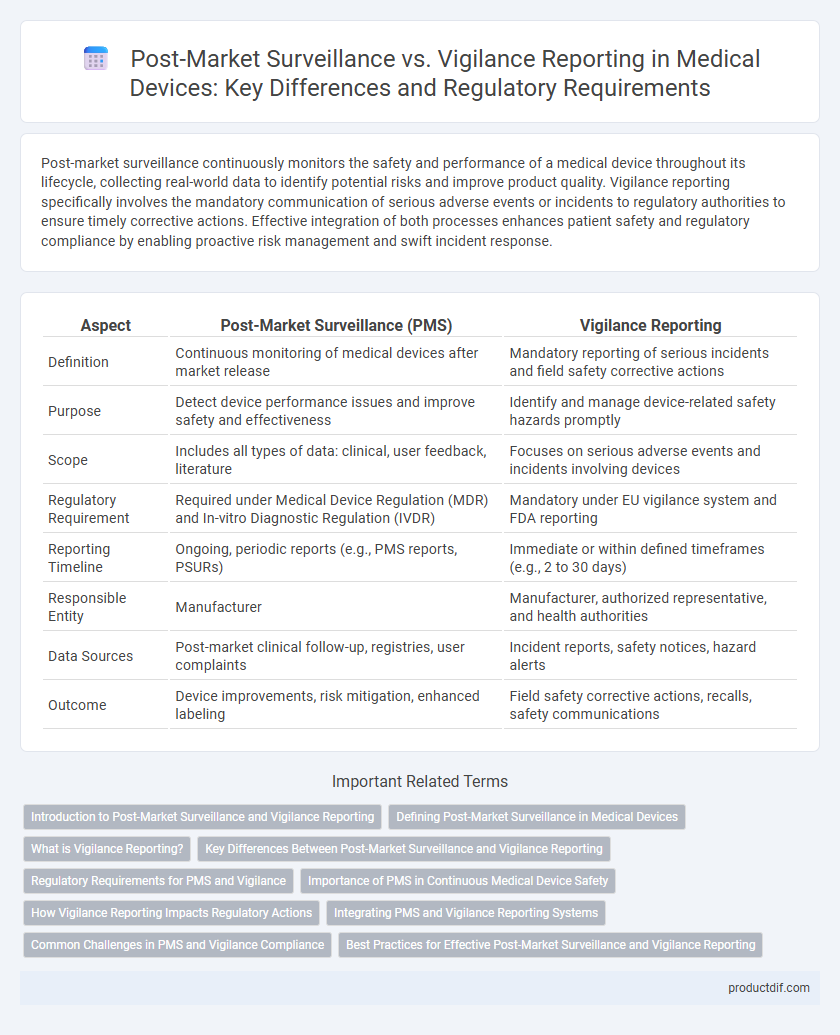
| Aspect | Post-Market Surveillance (PMS) | Vigilance Reporting |
|---|---|---|
| Definition | Continuous monitoring of medical devices after market release | Mandatory reporting of serious incidents and field safety corrective actions |
| Purpose | Detect device performance issues and improve safety and effectiveness | Identify and manage device-related safety hazards promptly |
| Scope | Includes all types of data: clinical, user feedback, literature | Focuses on serious adverse events and incidents involving devices |
| Regulatory Requirement | Required under Medical Device Regulation (MDR) and In-vitro Diagnostic Regulation (IVDR) | Mandatory under EU vigilance system and FDA reporting |
| Reporting Timeline | Ongoing, periodic reports (e.g., PMS reports, PSURs) | Immediate or within defined timeframes (e.g., 2 to 30 days) |
| Responsible Entity | Manufacturer | Manufacturer, authorized representative, and health authorities |
| Data Sources | Post-market clinical follow-up, registries, user complaints | Incident reports, safety notices, hazard alerts |
| Outcome | Device improvements, risk mitigation, enhanced labeling | Field safety corrective actions, recalls, safety communications |
Introduction to Post-Market Surveillance and Vigilance Reporting
Post-market surveillance involves the systematic collection and analysis of data on the safety and performance of medical devices after they have been released to the market, ensuring ongoing compliance with regulatory requirements. Vigilance reporting specifically refers to the mandatory process of documenting and reporting serious adverse events or device malfunctions that could potentially lead to health risks, enabling timely corrective actions. Both processes are critical for maintaining patient safety and improving device reliability through continuous monitoring and reporting protocols.
Defining Post-Market Surveillance in Medical Devices
Post-market surveillance in medical devices refers to the systematic collection, analysis, and monitoring of data related to device performance and safety after market approval. It encompasses ongoing activities to detect potential risks, performance issues, and adverse events to ensure continued compliance with regulatory standards. This process is broader than vigilance reporting, which specifically concerns the mandatory notification of serious incidents or adverse events to regulatory authorities.
What is Vigilance Reporting?
Vigilance reporting is a regulatory process that requires manufacturers and healthcare professionals to promptly report serious incidents and adverse events related to medical devices to authorities. This system ensures timely identification and correction of device-related hazards to protect patient safety. Vigilance reporting complements post-market surveillance by focusing specifically on critical risks and mandatory reporting obligations to regulatory bodies such as the FDA or the European Medicines Agency.
Key Differences Between Post-Market Surveillance and Vigilance Reporting
Post-market surveillance (PMS) involves systematic monitoring of medical devices' performance and safety throughout their lifecycle, capturing comprehensive data to ensure ongoing compliance and identify long-term risks. Vigilance reporting specifically addresses the mandatory notification of serious incidents, including device malfunctions or adverse events, to regulatory authorities within defined timeframes. The key difference lies in PMS's proactive, continuous data collection approach versus vigilance reporting's reactive, incident-driven communication focused on immediate safety concerns.
Regulatory Requirements for PMS and Vigilance
Post-market surveillance (PMS) mandates continuous monitoring of medical devices to ensure safety and performance as per regulatory frameworks like EU MDR 2017/745 and FDA 21 CFR Part 820. Vigilance reporting requires manufacturers to promptly report serious incidents or adverse events to regulatory authorities, fulfilling obligations under specific regulations such as the EU's Vigilance System and FDA's Medical Device Reporting (MDR). Compliance with PMS includes periodic safety update reports (PSURs), while vigilance reporting emphasizes timely notification and corrective actions to mitigate risks to patients.
Importance of PMS in Continuous Medical Device Safety
Post-market surveillance (PMS) plays a critical role in ensuring continuous medical device safety by systematically collecting and analyzing real-world data on device performance and adverse events. Unlike vigilance reporting, which focuses on mandatory notification of serious incidents, PMS provides a comprehensive framework for ongoing risk assessment and early detection of potential safety issues. Effective PMS strategies contribute to enhanced patient protection, regulatory compliance, and informed decision-making throughout the product lifecycle.
How Vigilance Reporting Impacts Regulatory Actions
Vigilance reporting provides critical, real-time data on adverse events associated with medical devices, enabling regulatory bodies to promptly assess risks and enforce corrective actions such as recalls or safety notices. This regulatory oversight ensures that emerging issues are systematically addressed, maintaining device safety and efficacy in the market. Post-market surveillance complements vigilance reporting by offering ongoing, comprehensive monitoring but vigilance reporting directly influences immediate regulatory decisions.
Integrating PMS and Vigilance Reporting Systems
Integrating Post-market Surveillance (PMS) and Vigilance Reporting systems enhances the monitoring of medical device performance by consolidating real-world data and adverse event reporting into a unified platform. This integration improves the timeliness and accuracy of detecting potential safety issues, facilitating rapid risk mitigation and regulatory compliance according to FDA and EU MDR requirements. Leveraging advanced data analytics and automated workflows supports proactive safety management and continuous device improvement throughout the product lifecycle.
Common Challenges in PMS and Vigilance Compliance
Post-market surveillance (PMS) and vigilance reporting in medical devices face common challenges such as data collection consistency, timely adverse event detection, and regulatory compliance across diverse markets. Ensuring robust traceability and effective risk management systems is critical to meet evolving global standards like MDR (EU Medical Device Regulation) and FDA guidelines. Inadequate signal detection and underreporting of incidents often compromise patient safety and delay corrective actions.
Best Practices for Effective Post-Market Surveillance and Vigilance Reporting
Effective post-market surveillance integrates continuous data collection on device performance, adverse events, and user feedback to proactively identify potential risks and ensure patient safety. Vigilance reporting mandates timely and accurate communication of serious incidents and corrective actions to regulatory authorities, facilitating rapid response and mitigation. Implementing automated monitoring systems, regular training for reporting staff, and maintaining robust documentation enhances compliance and supports a comprehensive risk management strategy.
Post-market surveillance vs Vigilance reporting Infographic
 productdif.com
productdif.com