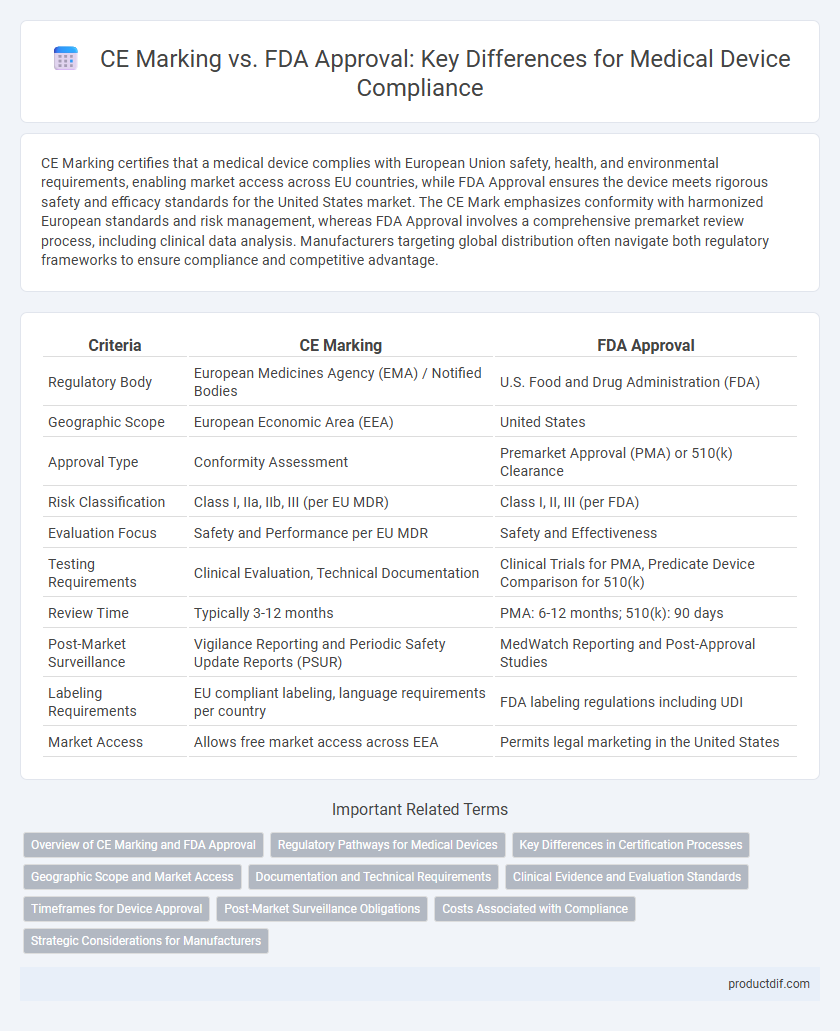
CE Marking certifies that a medical device complies with European Union safety, health, and environmental requirements, enabling market access across EU countries, while FDA Approval ensures the device meets rigorous safety and efficacy standards for the United States market. The CE Mark emphasizes conformity with harmonized European standards and risk management, whereas FDA Approval involves a comprehensive premarket review process, including clinical data analysis. Manufacturers targeting global distribution often navigate both regulatory frameworks to ensure compliance and competitive advantage.
Table of Comparison
| Criteria | CE Marking | FDA Approval |
|---|---|---|
| Regulatory Body | European Medicines Agency (EMA) / Notified Bodies | U.S. Food and Drug Administration (FDA) |
| Geographic Scope | European Economic Area (EEA) | United States |
| Approval Type | Conformity Assessment | Premarket Approval (PMA) or 510(k) Clearance |
| Risk Classification | Class I, IIa, IIb, III (per EU MDR) | Class I, II, III (per FDA) |
| Evaluation Focus | Safety and Performance per EU MDR | Safety and Effectiveness |
| Testing Requirements | Clinical Evaluation, Technical Documentation | Clinical Trials for PMA, Predicate Device Comparison for 510(k) |
| Review Time | Typically 3-12 months | PMA: 6-12 months; 510(k): 90 days |
| Post-Market Surveillance | Vigilance Reporting and Periodic Safety Update Reports (PSUR) | MedWatch Reporting and Post-Approval Studies |
| Labeling Requirements | EU compliant labeling, language requirements per country | FDA labeling regulations including UDI |
| Market Access | Allows free market access across EEA | Permits legal marketing in the United States |
Overview of CE Marking and FDA Approval
CE Marking certifies that a medical device complies with the European Union's Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR), allowing it to be marketed across EU member states. FDA Approval, managed by the U.S. Food and Drug Administration, requires rigorous evaluation of safety and effectiveness under the Federal Food, Drug, and Cosmetic Act (FD&C Act) before a device can enter the U.S. market. Both CE Marking and FDA Approval serve as regulatory benchmarks but differ significantly in assessment procedures, geographic scope, and post-market surveillance requirements.
Regulatory Pathways for Medical Devices
CE Marking and FDA approval represent distinct regulatory pathways for medical devices in Europe and the United States, respectively. CE Marking requires conformity with the EU Medical Device Regulation (MDR) or In Vitro Diagnostic Regulation (IVDR), emphasizing safety, performance, and clinical evaluation through notified bodies. FDA approval involves premarket notifications (510(k)), premarket approval (PMA), or de novo classification, focusing on rigorous evidence of safety and effectiveness based on the device risk class.
Key Differences in Certification Processes
CE Marking certifies medical devices for compliance with European Union regulations, focusing on safety, performance, and risk management outlined in the EU Medical Device Regulation (MDR). FDA Approval requires rigorous clinical trials and premarket submissions such as 510(k) or PMA to ensure safety and effectiveness for the US market, emphasizing regulatory scrutiny and data transparency. Both processes differ significantly in scope, timeline, and documentation requirements, making strategic regulatory planning essential for market entry.
Geographic Scope and Market Access
CE Marking certifies medical devices for the European Economic Area (EEA), enabling broad market access across all member countries under a unified regulatory framework. FDA Approval is specific to the United States market, where devices must meet stringent safety and efficacy standards dictated by the Food and Drug Administration before commercial distribution. Understanding these regulatory distinctions is crucial for manufacturers planning global market entry strategies, as CE Marking allows access to Europe while FDA Approval is mandatory for the U.S. and can influence acceptance in other regions.
Documentation and Technical Requirements
CE Marking for medical devices requires comprehensive technical documentation, including a Declaration of Conformity, risk management files, and clinical evaluation reports aligned with the Medical Device Regulation (MDR) 2017/745. FDA Approval mandates detailed submission of a premarket notification 510(k) or premarket approval (PMA), encompassing extensive clinical data, device description, and manufacturing information as specified by Title 21 CFR Part 807. Both regulatory pathways demand rigorous documentation demonstrating safety and efficacy, but CE Marking emphasizes conformity to European harmonized standards while FDA approval focuses on stringent U.S. regulatory compliance and evidence-based validation.
Clinical Evidence and Evaluation Standards
CE Marking requires demonstration of compliance with the European Medical Device Regulation (MDR), emphasizing clinical evaluation based on relevant clinical data and literature, supported by rigorous post-market surveillance. FDA Approval mandates substantive clinical evidence from well-controlled investigations, often requiring randomized clinical trials to establish safety and effectiveness according to stringent U.S. regulatory standards. Both frameworks prioritize patient safety but differ in clinical evidence requirements, evaluation methodologies, and regulatory scrutiny intensity.
Timeframes for Device Approval
CE Marking for medical devices generally requires a shorter approval timeframe, often ranging from a few months up to one year, depending on device classification and complexity. FDA approval processes tend to be lengthier, frequently extending from one to several years due to more rigorous clinical trial requirements and comprehensive review procedures. Manufacturers targeting both European and US markets must plan accordingly to accommodate these differing regulatory timeframes.
Post-Market Surveillance Obligations
CE Marking requires manufacturers to implement a continuous post-market surveillance system that collects and analyzes data on device performance and safety, enabling timely corrective actions in the European Union. FDA approval mandates comprehensive post-market surveillance including Medical Device Reporting (MDR), post-approval studies, and device tracking to monitor adverse events and ensure ongoing compliance in the United States. Both regulatory bodies emphasize proactive risk management and real-world evidence collection to maintain device safety and effectiveness throughout the product lifecycle.
Costs Associated with Compliance
CE Marking compliance typically incurs lower costs compared to FDA approval, as the European Union's regulatory process emphasizes conformity assessment through notified bodies, often resulting in reduced fees and shorter timelines. FDA approval involves extensive premarket submissions such as PMA or 510(k) processes, leading to higher expenses driven by clinical trials, documentation, and regulatory review. Manufacturers should budget for variable costs based on device classification, with FDA pathways generally demanding more substantial financial investment than CE Marking compliance.
Strategic Considerations for Manufacturers
Manufacturers targeting both the European and U.S. markets must navigate distinct regulatory frameworks, with CE Marking emphasizing compliance with the EU Medical Device Regulation (MDR) and FDA Approval requiring adherence to rigorous premarket notification or approval processes. Strategic considerations include timelines, costs, and clinical evidence requirements, as CE Marking often allows faster market access while FDA Approval demands more extensive documentation and validation. Aligning product development and quality management systems to meet both standards optimizes market reach, reduces regulatory risks, and enhances global competitiveness.
CE Marking vs FDA Approval Infographic
 productdif.com
productdif.com