Instructions for Use (IFU) provide healthcare professionals with detailed guidance on the proper operation, maintenance, and safety precautions of a medical device. Device for Use (DFU) typically refers to the actual medical device or software intended for clinical application, highlighting its practical deployment in patient care. Understanding the distinction between IFU as comprehensive documentation and DFU as the actionable device ensures compliance with regulatory standards and enhances patient safety.
Table of Comparison
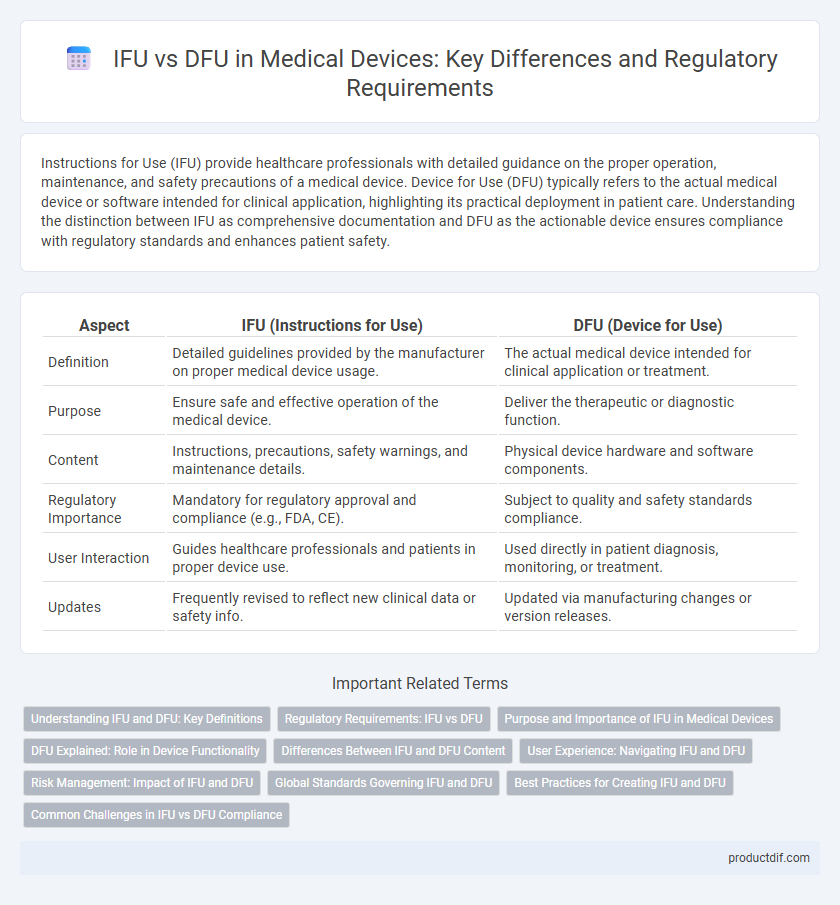
| Aspect | IFU (Instructions for Use) | DFU (Device for Use) |
|---|---|---|
| Definition | Detailed guidelines provided by the manufacturer on proper medical device usage. | The actual medical device intended for clinical application or treatment. |
| Purpose | Ensure safe and effective operation of the medical device. | Deliver the therapeutic or diagnostic function. |
| Content | Instructions, precautions, safety warnings, and maintenance details. | Physical device hardware and software components. |
| Regulatory Importance | Mandatory for regulatory approval and compliance (e.g., FDA, CE). | Subject to quality and safety standards compliance. |
| User Interaction | Guides healthcare professionals and patients in proper device use. | Used directly in patient diagnosis, monitoring, or treatment. |
| Updates | Frequently revised to reflect new clinical data or safety info. | Updated via manufacturing changes or version releases. |
Understanding IFU and DFU: Key Definitions
Instructions for Use (IFU) and Device Follow-Up (DFU) are critical components in medical device management, with IFU providing detailed guidelines on device operation, safety, and maintenance, ensuring regulatory compliance and user safety. DFU involves systematic monitoring and evaluation post-device implementation to assess performance, identify adverse events, and support clinical decision-making. Understanding these definitions enhances device lifecycle management and patient outcomes in healthcare settings.
Regulatory Requirements: IFU vs DFU
Regulatory requirements for Instructions for Use (IFU) and Device Follow-Up (DFU) differ significantly in medical device compliance. IFUs must comply with strict guidelines outlined by regulatory bodies such as the FDA and EU MDR, ensuring clear communication of safe usage, warnings, indications, and contraindications to users. DFU protocols focus on post-market surveillance, requiring manufacturers to collect and analyze safety and performance data to meet ongoing regulatory obligations for device monitoring and risk management.
Purpose and Importance of IFU in Medical Devices
Instructions for Use (IFU) are essential documents that provide detailed guidance on the safe and effective use of medical devices, ensuring compliance with regulatory standards such as FDA and ISO 13485. Unlike Device Field Updates (DFU), which inform users about modifications or safety concerns, IFUs serve as the primary source of information for device operation, maintenance, and troubleshooting. Properly designed IFUs minimize user errors, enhance patient safety, and support healthcare professionals in achieving optimal clinical outcomes.
DFU Explained: Role in Device Functionality
Device Functionality Understanding (DFU) is a crucial process that ensures medical devices operate properly through firmware updates and diagnostics. Unlike Instructions for Use (IFU), which provide end-user guidelines, DFU focuses on maintaining and enhancing device performance by managing software and hardware integration. Effective DFU enables seamless device functionality improvements, critical for patient safety and device reliability.
Differences Between IFU and DFU Content
Instructions for Use (IFU) provide comprehensive guidance on the safe and effective operation, maintenance, and troubleshooting of a medical device, including detailed procedures, warnings, and precautions. Device Family User Guide (DFU) offers broader information applicable to an entire family of related devices, highlighting common features, general usage principles, and compatibility guidelines. The key difference lies in IFU's specificity to a single device model versus DFU's collective approach for multiple device variants within the same product line.
User Experience: Navigating IFU and DFU
Navigating Instructions for Use (IFU) and Device Firmware Updates (DFU) is crucial for enhancing medical device user experience by ensuring clarity and accessibility in operational guidance and device maintenance. IFUs provide comprehensive procedural and safety information essential for correct device operation, while DFUs focus on software enhancements that improve device performance and security. Streamlined integration of both IFU and DFU materials reduces user confusion and supports adherence to regulatory standards, ultimately optimizing device reliability and patient safety.
Risk Management: Impact of IFU and DFU
Instructions for Use (IFU) and Device Failure Updates (DFU) are critical components in medical device risk management, directly influencing user safety and device performance. Properly maintained IFUs provide clear operational guidelines that reduce misuse risks, while timely DFUs address device malfunctions to prevent adverse events. Integrating both ensures comprehensive risk mitigation by aligning user instructions with real-world device performance updates.
Global Standards Governing IFU and DFU
Global standards governing Instructions for Use (IFU) and Device for Use (DFU) ensure safety, compliance, and effectiveness across medical device markets. Key regulatory frameworks include the FDA's 21 CFR Part 820 and ISO 14971 for risk management, alongside the European MDR 2017/745, which mandates clarity, language accessibility, and comprehensive labeling in IFU and DFU documents. Harmonization efforts by the International Medical Device Regulators Forum (IMDRF) promote consistent global requirements for usability, content structure, and risk communication in IFU and DFU.
Best Practices for Creating IFU and DFU
Creating effective Instructions for Use (IFU) and Device Field Updates (DFU) requires clear, concise language tailored to the end user's technical proficiency and regulatory standards such as FDA 21 CFR Part 820. Best practices include incorporating detailed step-by-step procedures, visual aids, and troubleshooting tips to enhance user comprehension and safety. Regular review and validation of both IFU and DFU ensure compliance with evolving medical device regulations and improve overall device performance in clinical settings.
Common Challenges in IFU vs DFU Compliance
Common challenges in IFU (Instructions for Use) versus DFU (Directions for Use) compliance include ensuring clarity and comprehensiveness to prevent misuse of medical devices. Regulatory requirements often demand meticulous accuracy in both IFU and DFU documentation to avoid discrepancies that could lead to safety risks or product recalls. Managing multilingual translations and updates further complicates adherence to evolving standards in medical device regulations.
IFU vs DFU Infographic
 productdif.com
productdif.com