Labeling requirements for medical devices mandate clear identification, safety information, and regulatory compliance details on the product packaging to ensure user safety and traceability. Instructions for Use (IFU) provide comprehensive guidance on device operation, maintenance, and troubleshooting to enable proper usage and minimize risk. Both labeling and IFU must adhere to stringent regulatory standards to guarantee effective communication and patient safety.
Table of Comparison
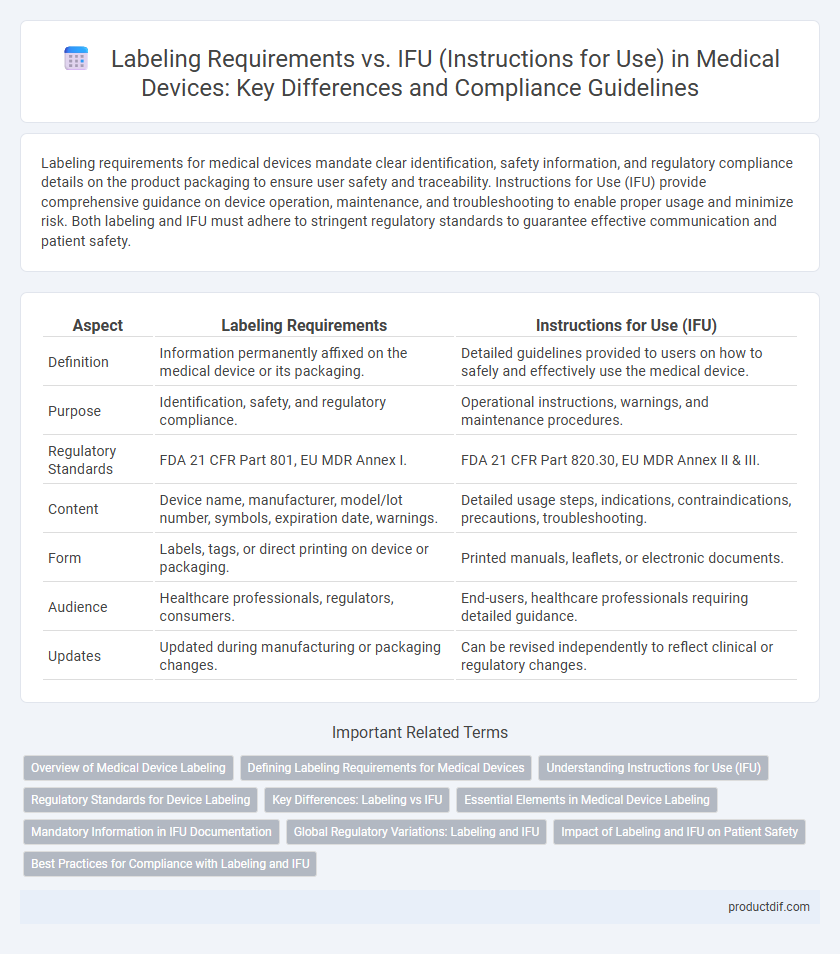
| Aspect | Labeling Requirements | Instructions for Use (IFU) |
|---|---|---|
| Definition | Information permanently affixed on the medical device or its packaging. | Detailed guidelines provided to users on how to safely and effectively use the medical device. |
| Purpose | Identification, safety, and regulatory compliance. | Operational instructions, warnings, and maintenance procedures. |
| Regulatory Standards | FDA 21 CFR Part 801, EU MDR Annex I. | FDA 21 CFR Part 820.30, EU MDR Annex II & III. |
| Content | Device name, manufacturer, model/lot number, symbols, expiration date, warnings. | Detailed usage steps, indications, contraindications, precautions, troubleshooting. |
| Form | Labels, tags, or direct printing on device or packaging. | Printed manuals, leaflets, or electronic documents. |
| Audience | Healthcare professionals, regulators, consumers. | End-users, healthcare professionals requiring detailed guidance. |
| Updates | Updated during manufacturing or packaging changes. | Can be revised independently to reflect clinical or regulatory changes. |
Overview of Medical Device Labeling
Medical device labeling encompasses all information accompanying the device, including packaging labels, tags, and inserts, ensuring compliance with regulatory standards such as FDA 21 CFR Part 801 and EU MDR 2017/745. Instructions for Use (IFU) provide detailed guidance on safe operation, maintenance, and troubleshooting, forming a critical subset of the overall labeling requirements. Accurate and clear labeling improves device safety, usability, and regulatory compliance, reducing risks associated with misuse or misunderstanding.
Defining Labeling Requirements for Medical Devices
Labeling requirements for medical devices encompass all mandatory information that must be clearly displayed on the device or its packaging to ensure patient safety and regulatory compliance. This includes device identification, manufacturer details, lot or serial numbers, expiration dates, and essential safety warnings, which differ from the more detailed and comprehensive Instructions for Use (IFU) that provide step-by-step guidance for proper device operation. Regulatory authorities such as the FDA and EU MDR specify precise labeling content to promote traceability, risk communication, and correct device usage, making accurate labeling a critical aspect of medical device approval and market access.
Understanding Instructions for Use (IFU)
Understanding Instructions for Use (IFU) is crucial for ensuring the safe and effective operation of medical devices, as these documents provide detailed guidance on device handling, operation, and maintenance. IFUs contain specific information such as intended use, contraindications, warnings, and troubleshooting steps, which differ from basic labeling that generally includes product identification and regulatory markings. Clear comprehension of IFUs helps healthcare professionals minimize risks, comply with regulatory standards, and optimize patient outcomes through proper device utilization.
Regulatory Standards for Device Labeling
Medical device labeling must comply with regulatory standards such as FDA 21 CFR Part 801 and EU MDR 2017/745, ensuring accurate, clear, and comprehensive information for safe device use. Labeling requirements include essential device identifiers, manufacturer details, and safety warnings, while the Instructions for Use (IFU) provide detailed use protocols, maintenance guidance, and troubleshooting instructions. Harmonizing labeling and IFU content is critical for regulatory approval and patient safety across global markets.
Key Differences: Labeling vs IFU
Labeling requirements for medical devices focus on the information directly printed or attached to the product or its packaging, including identification, manufacturer details, lot numbers, and basic safety warnings. Instructions for Use (IFU) provide detailed guidance on device operation, maintenance, contraindications, and troubleshooting to ensure safe and effective use by healthcare professionals or patients. The key difference lies in labeling offering essential, concise data for product identification and safety, while IFU contains comprehensive instructions supporting proper device utilization.
Essential Elements in Medical Device Labeling
Essential elements in medical device labeling include the device name, manufacturer information, lot or serial number, and expiration date to ensure traceability and compliance. Instructions for Use (IFU) provide detailed guidance on device operation, safety warnings, contraindications, and maintenance which are critical for user safety and regulatory adherence. Clear differentiation between labeling and IFU content optimizes usability and regulatory compliance in the medical device industry.
Mandatory Information in IFU Documentation
Mandatory information in IFU documentation for medical devices includes detailed device description, intended use, contraindications, warnings, and step-by-step usage instructions critical for safe operation. Regulatory agencies such as the FDA and EU MDR specify precise labeling requirements to ensure the IFU provides comprehensive safety and performance data. Accurate inclusion of these elements minimizes user error and ensures compliance with international standards like ISO 13485.
Global Regulatory Variations: Labeling and IFU
Global regulatory variations in medical device labeling and Instructions for Use (IFU) primarily reflect diverse language requirements, content specificity, and compliance standards mandated by authorities such as the FDA (USA), MDR (EU), and PMDA (Japan). Labeling must include essential information like device identification, safety warnings, and manufacturer details, while IFU provide detailed operational guidance, contraindications, and troubleshooting aligned with regional norms. Harmonizing these elements requires understanding country-specific mandates under frameworks like ISO 13485 and global standards from the International Medical Device Regulators Forum (IMDRF).
Impact of Labeling and IFU on Patient Safety
Clear and accurate labeling combined with comprehensive Instructions for Use (IFU) significantly enhances patient safety by minimizing misuse and ensuring proper device operation. Regulatory standards like FDA 21 CFR Part 801 and ISO 13485 mandate precise labeling and IFU to provide critical information on device indications, contraindications, warnings, and maintenance. Effective integration of labeling and IFU reduces the risk of user error, thereby improving clinical outcomes and adherence to safety protocols.
Best Practices for Compliance with Labeling and IFU
Ensuring compliance with medical device labeling requirements and Instructions for Use (IFU) involves clearly differentiating mandatory information such as device identification, safety warnings, and regulatory symbols on the label from detailed operational guidance provided in the IFU. Best practices include using plain language, incorporating standardized symbols, and validating content accuracy through user testing to enhance readability and minimize user errors. Maintaining up-to-date FDA and EU MDR guidelines alignment throughout the labeling and IFU documentation process strengthens regulatory compliance and patient safety.
Labeling Requirements vs IFU (Instructions for Use) Infographic
 productdif.com
productdif.com