Breakthrough devices offer innovative solutions that address unmet medical needs, often providing faster access to advanced technology through expedited regulatory pathways. Standard devices follow traditional approval processes and typically represent established technologies with proven safety and efficacy profiles. Choosing a breakthrough device can significantly impact patient outcomes by enabling earlier intervention with cutting-edge treatment options.
Table of Comparison
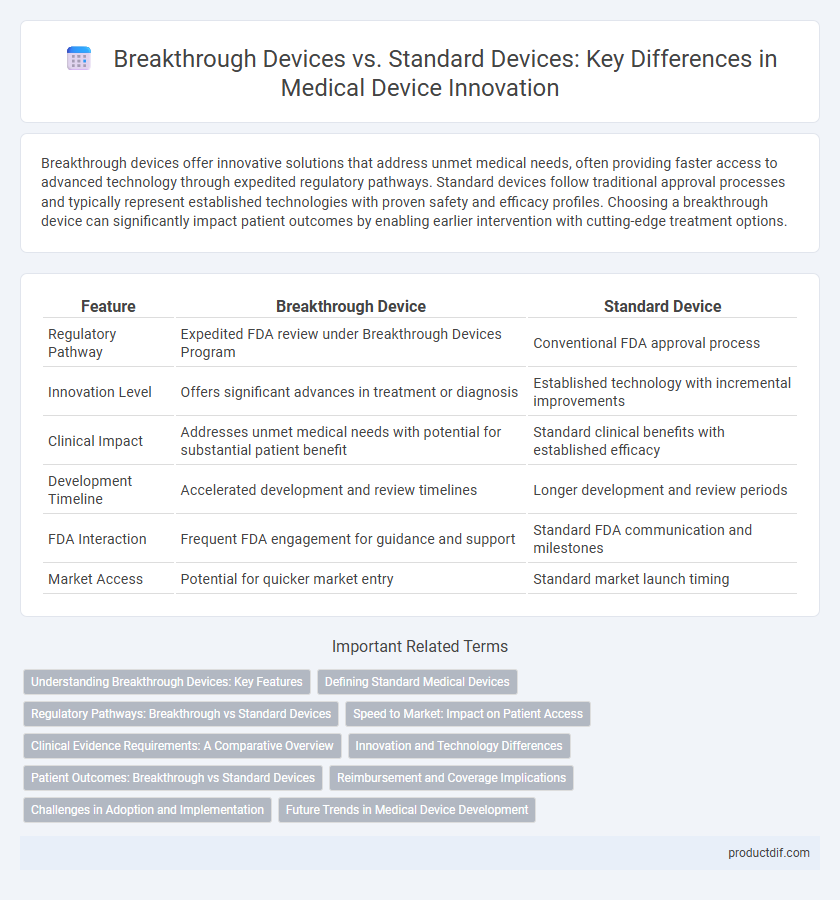
| Feature | Breakthrough Device | Standard Device |
|---|---|---|
| Regulatory Pathway | Expedited FDA review under Breakthrough Devices Program | Conventional FDA approval process |
| Innovation Level | Offers significant advances in treatment or diagnosis | Established technology with incremental improvements |
| Clinical Impact | Addresses unmet medical needs with potential for substantial patient benefit | Standard clinical benefits with established efficacy |
| Development Timeline | Accelerated development and review timelines | Longer development and review periods |
| FDA Interaction | Frequent FDA engagement for guidance and support | Standard FDA communication and milestones |
| Market Access | Potential for quicker market entry | Standard market launch timing |
Understanding Breakthrough Devices: Key Features
Breakthrough devices are medical devices that provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases, offering significant advantages over standard devices. These devices often incorporate innovative technology, demonstrating substantial improvement in safety and effectiveness, which accelerates their approval process by regulatory authorities like the FDA. Key features include expedited review pathways, enhanced patient outcomes, and the potential to address unmet medical needs more efficiently than traditional medical devices.
Defining Standard Medical Devices
Standard medical devices are products that have received regulatory approval based on established safety and effectiveness criteria without qualifying for the Breakthrough Device designation. These devices typically undergo conventional review pathways, demonstrating compliance with existing standards set by agencies such as the FDA. Unlike Breakthrough Devices, standard devices do not require expedited processes and focus on proven technology with well-documented clinical outcomes.
Regulatory Pathways: Breakthrough vs Standard Devices
Breakthrough devices benefit from expedited regulatory pathways such as the FDA's Breakthrough Devices Program, which facilitates faster review and access through priority review and interactive communication with regulators. Standard devices follow traditional pathways like the 510(k) premarket notification or Premarket Approval (PMA), often involving longer review times and more rigid submission requirements. This difference accelerates market entry for breakthrough devices addressing unmet medical needs or presenting significant clinical advantages over existing alternatives.
Speed to Market: Impact on Patient Access
Breakthrough devices receive prioritized FDA review, significantly reducing approval time compared to standard devices, accelerating their market entry. Faster regulatory clearance enhances timely patient access to innovative treatments, often addressing unmet medical needs more quickly. This expedited pathway can improve clinical outcomes by enabling earlier adoption of cutting-edge medical technologies.
Clinical Evidence Requirements: A Comparative Overview
Breakthrough devices require robust clinical evidence demonstrating substantial improvement over existing treatments, often supported by early feasibility studies and real-world data to expedite patient access. Standard devices typically adhere to established regulatory pathways with well-defined clinical trial phases, emphasizing safety and efficacy through randomized controlled trials. The emphasis on clinical evidence for breakthrough devices prioritizes faster validation of innovative benefits while maintaining rigorous safety standards.
Innovation and Technology Differences
Breakthrough devices incorporate cutting-edge technologies such as AI algorithms, advanced sensors, or novel biomaterials that significantly enhance diagnostic accuracy and therapeutic outcomes compared to standard devices. These innovations enable faster regulatory pathways and often provide treatments for conditions with no existing alternatives, reflecting a higher level of technological advancement and clinical impact. Standard devices, while effective, typically rely on established technologies and incremental improvements, lacking the transformative features present in breakthrough innovations.
Patient Outcomes: Breakthrough vs Standard Devices
Breakthrough medical devices demonstrate significantly improved patient outcomes by offering innovative treatments that address unmet clinical needs, often resulting in faster recovery times and reduced complications. Standard devices provide established, reliable solutions but may lack the advanced capabilities found in breakthrough technologies, potentially limiting optimal patient benefits. Clinical studies indicate breakthrough devices can enhance quality of life and overall health outcomes compared to traditional options.
Reimbursement and Coverage Implications
Breakthrough devices often receive expedited FDA approval, enhancing market access and increasing reimbursement opportunities from Medicare and private insurers compared to standard devices. Coverage implications favor breakthrough devices due to their innovative nature and potential for improved patient outcomes, leading to higher likelihood of favorable coding and payment policies. Standard devices typically face longer reimbursement review periods and may require additional evidence to achieve similar coverage levels.
Challenges in Adoption and Implementation
Breakthrough medical devices face significant challenges in adoption and implementation due to regulatory uncertainties, higher costs, and limited clinical evidence compared to standard devices. Healthcare providers often encounter difficulties integrating breakthrough technologies into existing workflows, resulting in slower acceptance and reimbursement hurdles. Moreover, standard devices benefit from established user familiarity and well-defined protocols, creating resistance to change and limiting the scalability of innovative solutions.
Future Trends in Medical Device Development
Breakthrough devices leverage advanced technologies such as AI integration and personalized medicine to offer enhanced diagnostic accuracy and therapeutic outcomes compared to standard devices. Future trends emphasize the incorporation of real-time data analytics, remote patient monitoring, and miniaturized, wearable designs to improve patient engagement and treatment efficiency. Regulatory frameworks are evolving to accelerate the approval process, fostering innovation and faster market access for transformative medical technologies.
Breakthrough Device vs Standard Device Infographic
 productdif.com
productdif.com