ISO 13485 specifies requirements for quality management systems in the design and manufacture of medical devices, ensuring compliance with regulatory standards. ISO 14971 focuses on risk management, providing a framework for identifying, evaluating, and controlling risks associated with medical devices throughout their lifecycle. Both standards are essential for ensuring product safety and effectiveness but address different aspects of medical device development and maintenance.
Table of Comparison
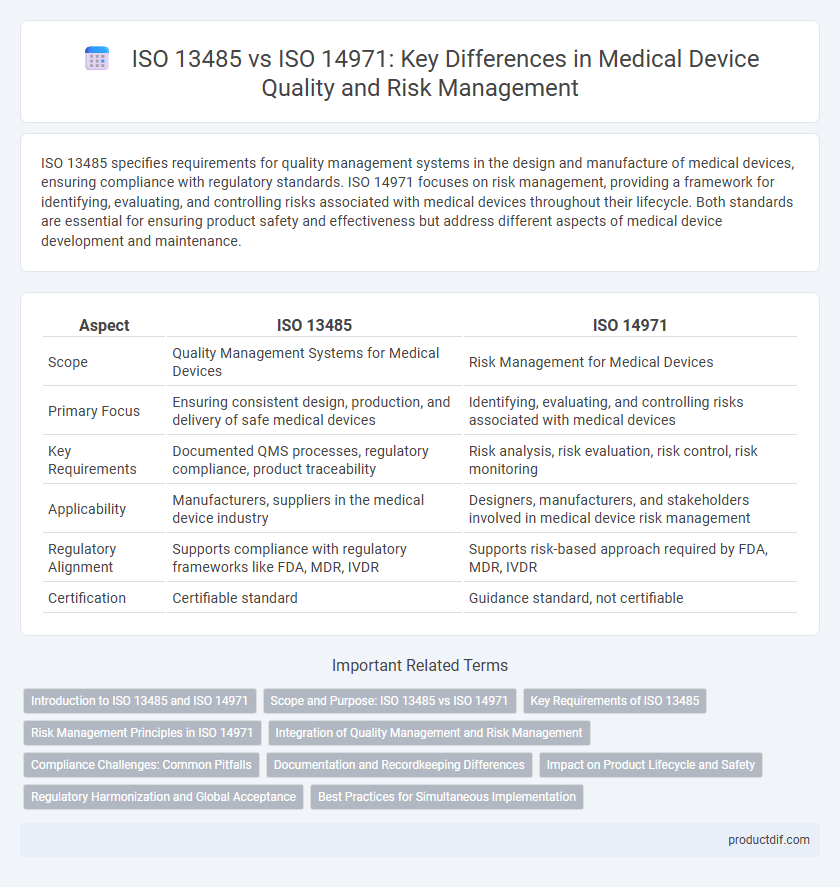
| Aspect | ISO 13485 | ISO 14971 |
|---|---|---|
| Scope | Quality Management Systems for Medical Devices | Risk Management for Medical Devices |
| Primary Focus | Ensuring consistent design, production, and delivery of safe medical devices | Identifying, evaluating, and controlling risks associated with medical devices |
| Key Requirements | Documented QMS processes, regulatory compliance, product traceability | Risk analysis, risk evaluation, risk control, risk monitoring |
| Applicability | Manufacturers, suppliers in the medical device industry | Designers, manufacturers, and stakeholders involved in medical device risk management |
| Regulatory Alignment | Supports compliance with regulatory frameworks like FDA, MDR, IVDR | Supports risk-based approach required by FDA, MDR, IVDR |
| Certification | Certifiable standard | Guidance standard, not certifiable |
Introduction to ISO 13485 and ISO 14971
ISO 13485 specifies requirements for a quality management system specific to the medical device industry, emphasizing consistent design, development, production, and delivery of safe medical devices. ISO 14971 focuses on risk management principles and processes for identifying hazards, estimating and evaluating risks, controlling them, and monitoring the effectiveness of controls throughout the medical device lifecycle. Together, ISO 13485 and ISO 14971 provide a comprehensive framework ensuring regulatory compliance, product safety, and risk mitigation in medical device manufacturing.
Scope and Purpose: ISO 13485 vs ISO 14971
ISO 13485 specifies requirements for a quality management system focused on the design, production, and distribution of medical devices, ensuring consistent product safety and regulatory compliance. ISO 14971 outlines a risk management process specifically for identifying, evaluating, and controlling risks associated with medical devices throughout their lifecycle. While ISO 13485 addresses overall quality assurance, ISO 14971 emphasizes risk assessment and mitigation to enhance patient safety and product reliability.
Key Requirements of ISO 13485
ISO 13485 mandates comprehensive quality management system requirements for medical device manufacturers, emphasizing risk management, process control, and regulatory compliance. It requires documented procedures for production, traceability, and corrective actions to ensure consistent product safety and effectiveness throughout the device lifecycle. This standard integrates with ISO 14971 by supporting systematic risk management but centers on quality management system implementation rather than risk analysis alone.
Risk Management Principles in ISO 14971
ISO 13485 sets comprehensive requirements for a quality management system specific to medical devices, while ISO 14971 focuses exclusively on risk management principles throughout the product lifecycle. ISO 14971 mandates systematic identification, analysis, evaluation, control, and monitoring of risks associated with medical devices to ensure patient safety and regulatory compliance. Implementing ISO 14971 within an ISO 13485 framework enhances risk-based decision-making and supports continuous improvement in medical device manufacturing processes.
Integration of Quality Management and Risk Management
ISO 13485 establishes comprehensive requirements for a Quality Management System specific to medical devices, ensuring consistent product quality and regulatory compliance. ISO 14971 focuses exclusively on Risk Management, guiding the identification, evaluation, and control of risks associated with medical devices throughout their lifecycle. Integrating ISO 14971 risk management processes within the ISO 13485 framework enhances overall product safety and efficacy by embedding risk controls directly into quality management practices.
Compliance Challenges: Common Pitfalls
Compliance challenges in medical device regulation often arise from misinterpreting the distinct scopes of ISO 13485 and ISO 14971. ISO 13485 focuses on quality management systems specifically for medical devices, emphasizing consistent product design and manufacturing processes, while ISO 14971 centers on risk management throughout the device lifecycle. Common pitfalls include inadequate integration of risk assessment procedures from ISO 14971 into the quality management framework required by ISO 13485, leading to gaps in risk control and documentation compliance.
Documentation and Recordkeeping Differences
ISO 13485 emphasizes comprehensive documentation and recordkeeping to ensure consistent quality management throughout the medical device lifecycle, including design, production, and post-market activities. ISO 14971 focuses on documenting risk management processes, such as hazard identification, risk assessment, control measures, and residual risk evaluation to ensure patient safety. While ISO 13485 mandates maintaining quality system records, ISO 14971 requires detailed risk files that integrate risk analysis results with design and clinical evidence documentation.
Impact on Product Lifecycle and Safety
ISO 13485 focuses on quality management systems specific to medical devices, ensuring consistent product design, development, manufacturing, and post-market surveillance, which directly enhances product lifecycle management. ISO 14971 centers on risk management for medical devices, identifying hazards, estimating and evaluating risks, and implementing controls to maintain patient safety throughout the entire product lifecycle. Integrating ISO 13485 with ISO 14971 ensures a comprehensive approach where risk controls are embedded into quality processes, significantly improving device safety and regulatory compliance.
Regulatory Harmonization and Global Acceptance
ISO 13485 specifies requirements for a comprehensive quality management system in the medical device industry, ensuring consistent product safety and regulatory compliance across global markets. ISO 14971 focuses on risk management processes to identify, assess, and mitigate risks associated with medical devices, promoting patient safety and regulatory risk assessment standards. Together, these standards support regulatory harmonization and facilitate global acceptance by aligning quality management and risk management practices with international regulatory requirements.
Best Practices for Simultaneous Implementation
Implementing ISO 13485 and ISO 14971 simultaneously enhances medical device quality management and risk management efficiency by integrating process controls with risk assessment protocols. Key best practices include aligning documentation to meet both quality system requirements and risk management clauses, fostering cross-functional teamwork to streamline compliance efforts, and utilizing software tools that support concurrent management of quality and risk data. Maintaining a unified training program ensures consistent understanding of regulatory expectations across design, production, and post-market surveillance activities.
ISO 13485 vs ISO 14971 Infographic
 productdif.com
productdif.com