EUDAMED provides a centralized European database for medical device regulatory information, enhancing transparency and traceability across EU member states. GUDID serves as the United States' repository for device identification, supporting unique device identification (UDI) compliance and facilitating device tracking. Both systems improve patient safety and regulatory oversight but differ in geographic scope and specific regulatory requirements.
Table of Comparison
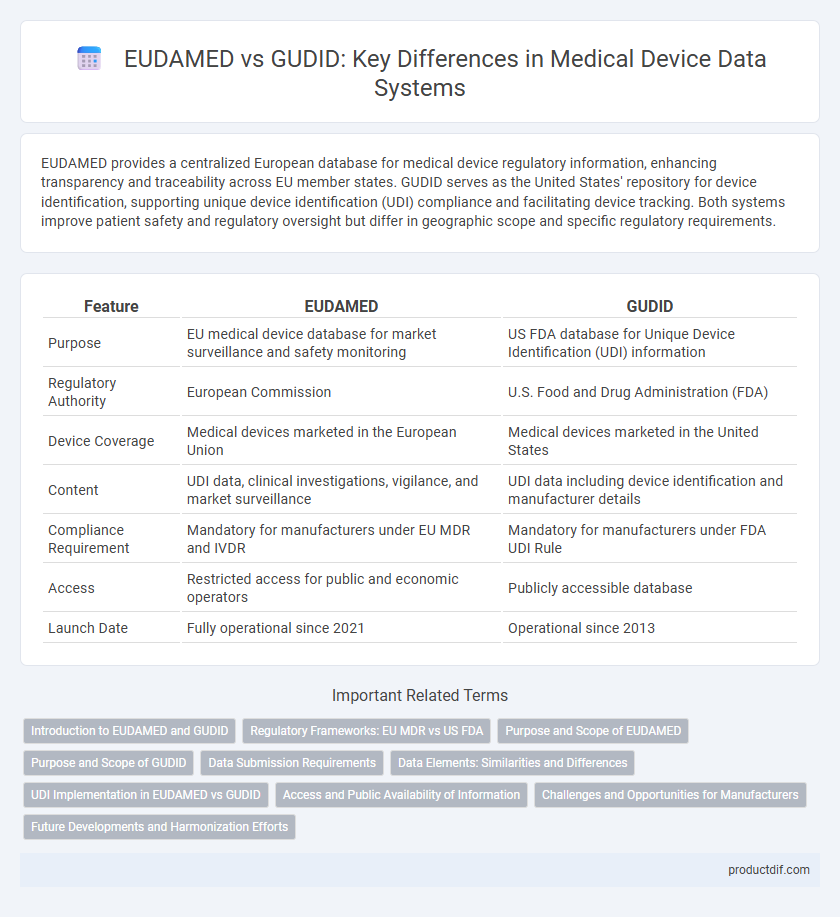
| Feature | EUDAMED | GUDID |
|---|---|---|
| Purpose | EU medical device database for market surveillance and safety monitoring | US FDA database for Unique Device Identification (UDI) information |
| Regulatory Authority | European Commission | U.S. Food and Drug Administration (FDA) |
| Device Coverage | Medical devices marketed in the European Union | Medical devices marketed in the United States |
| Content | UDI data, clinical investigations, vigilance, and market surveillance | UDI data including device identification and manufacturer details |
| Compliance Requirement | Mandatory for manufacturers under EU MDR and IVDR | Mandatory for manufacturers under FDA UDI Rule |
| Access | Restricted access for public and economic operators | Publicly accessible database |
| Launch Date | Fully operational since 2021 | Operational since 2013 |
Introduction to EUDAMED and GUDID
EUDAMED (European Database on Medical Devices) serves as the centralized repository for medical device data within the European Union, enhancing transparency and regulatory oversight under the EU Medical Device Regulation (MDR). GUDID (Global Unique Device Identification Database) operates as the United States' FDA-managed system to catalog device identification information for medical devices marketed domestically. Both databases facilitate device traceability, with EUDAMED emphasizing compliance with European regulatory frameworks and GUDID focusing on the implementation of Unique Device Identification (UDI) systems in the U.S. market.
Regulatory Frameworks: EU MDR vs US FDA
EUDAMED, established under the European Union Medical Device Regulation (EU MDR), serves as a central database enhancing transparency and regulatory oversight for medical devices in the EU, including device registration, clinical investigations, and post-market surveillance. The US FDA mandates the use of the Global Unique Device Identification Database (GUDID) to maintain device identification data for all medical devices marketed in the United States, facilitating traceability and adverse event reporting. Both frameworks emphasize device safety and traceability but differ in scope and regulatory requirements, with EU MDR imposing broader clinical evidence obligations and EUDAMED integrating multiple regulatory modules.
Purpose and Scope of EUDAMED
EUDAMED serves as the European Union's comprehensive database designed to enhance medical device transparency, safety, and regulatory compliance by integrating information on devices, economic operators, clinical investigations, and vigilance activities across member states. Its scope covers all medical devices and in vitro diagnostic devices marketed within the EU, facilitating traceability and post-market surveillance. In contrast, the Global Unique Device Identification Database (GUDID) primarily functions as a U.S. FDA-managed repository focused on device identification to support device tracking and patient safety within the American healthcare system.
Purpose and Scope of GUDID
The Global Unique Device Identification Database (GUDID) serves as a centralized repository managed by the FDA to store key identification data for medical devices marketed in the United States, enabling traceability and enhancing post-market surveillance. In contrast, EUDAMED, established by the European Medicines Agency, encompasses a broader regulatory scope covering device registration, economic operators, clinical investigations, and vigilance within the European Union. GUDID specifically focuses on the Unique Device Identifier (UDI) system to improve patient safety and streamline device recalls, while EUDAMED integrates multiple modules to support comprehensive conformity assessment and transparency across all EU member states.
Data Submission Requirements
EUDAMED requires comprehensive data submission covering device registration, clinical investigations, and vigilance reports to ensure compliance with the EU Medical Device Regulation (MDR). GUDID focuses specifically on unique device identification (UDI) data, mandating submission of device identifiers, manufacturer details, and packaging information to support traceability in the U.S. market under FDA regulations. Both databases demand accurate, standardized data but differ in scope: EUDAMED integrates multiple regulatory facets, while GUDID centers on UDI data for device identification and tracking.
Data Elements: Similarities and Differences
EUDAMED and GUDID both serve as regulatory databases for medical device information but differ in scope and data elements. EUDAMED covers detailed data including actor registration, device identification, clinical investigations, and vigilance, while GUDID primarily focuses on Unique Device Identifiers (UDI) and product attributes for devices marketed in the United States. Both databases require device identification data, though EUDAMED includes broader regulatory and performance-related information aligned with the EU MDR, contrasting with GUDID's emphasis on supply chain and traceability details under the FDA.
UDI Implementation in EUDAMED vs GUDID
EUDAMED and GUDID serve as central databases for Unique Device Identification (UDI) implementation but differ in regulatory scope and data structure. EUDAMED supports the European Union Medical Device Regulation (EU MDR) with comprehensive data on device registration, economic operators, and certificates, while GUDID is a U.S. FDA database focusing primarily on the submission of UDI device identification attributes. Effective UDI implementation requires manufacturers to comply with EUDAMED for EU market access and GUDID for U.S. regulatory requirements, ensuring traceability and post-market surveillance.
Access and Public Availability of Information
EUDAMED provides comprehensive access to detailed information on medical devices marketed in the European Union, enhancing transparency through public databases covering device registration, performance, and vigilance data. GUDID, managed by the U.S. FDA, serves as a centralized repository for Unique Device Identification (UDI) information, ensuring public availability of device identification and manufacturer data primarily within the American regulatory framework. Both systems promote traceability and patient safety by making critical device information accessible to healthcare professionals, regulators, and the public, but EUDAMED offers broader data scope, while GUDID focuses specifically on identification details.
Challenges and Opportunities for Manufacturers
EUDAMED and GUDID present unique challenges and opportunities for medical device manufacturers, with EUDAMED requiring comprehensive compliance with EU MDR regulations and extensive data reporting to ensure transparency in the European market. GUDID, managed by the FDA, demands accurate submission of Device Identifier (DI) information, enhancing traceability and patient safety in the US market. Manufacturers face the opportunity to streamline regulatory processes and improve global market access by effectively managing data integration and ensuring regulatory alignment between these two critical databases.
Future Developments and Harmonization Efforts
Future developments in medical device regulation emphasize enhanced interoperability between EUDAMED and GUDID to streamline data exchange and improve traceability across global markets. Harmonization efforts focus on aligning data standards, such as Unique Device Identification (UDI) formats, to facilitate regulatory convergence between the European Union and the United States. These initiatives aim to reduce compliance burdens for manufacturers while strengthening patient safety through more robust and transparent device databases.
EUDAMED vs GUDID Infographic
 productdif.com
productdif.com