A predicate device is an FDA-cleared medical device used as a benchmark to demonstrate substantial equivalence for new device approval under the 510(k) pathway. In contrast, a reference device serves as a comparison tool primarily in clinical evaluations or performance testing but may not hold FDA clearance status. Understanding the distinction between predicate and reference devices is critical for regulatory strategy and market entry planning in medical device development.
Table of Comparison
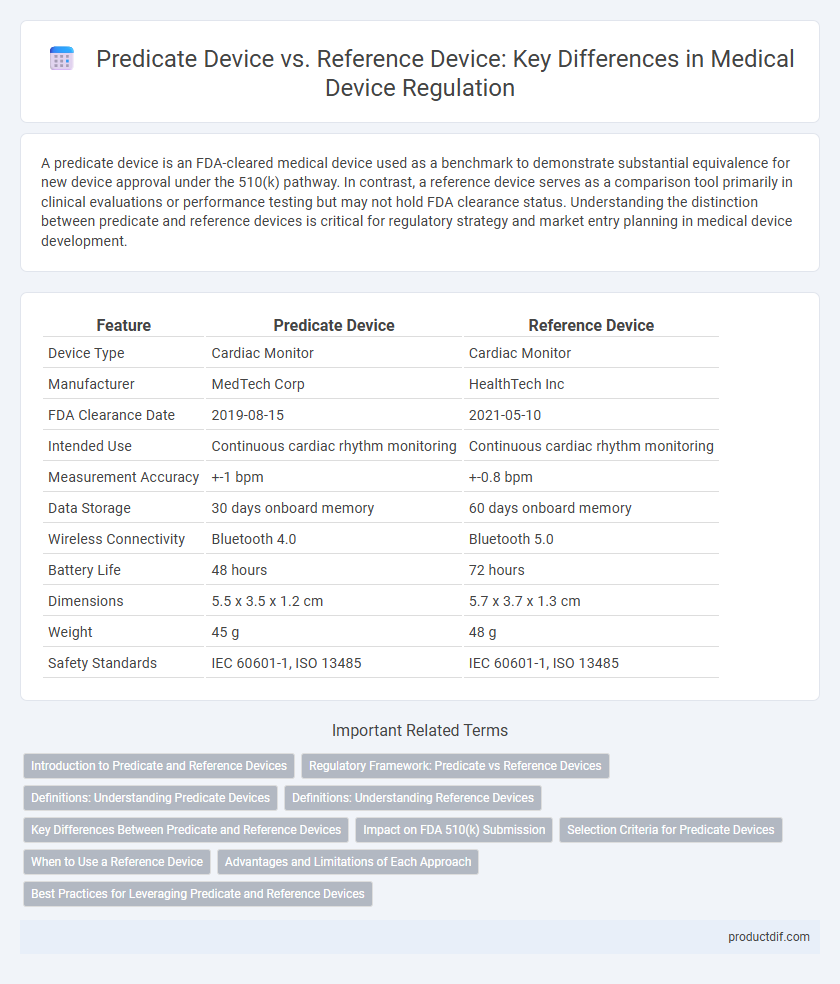
| Feature | Predicate Device | Reference Device |
|---|---|---|
| Device Type | Cardiac Monitor | Cardiac Monitor |
| Manufacturer | MedTech Corp | HealthTech Inc |
| FDA Clearance Date | 2019-08-15 | 2021-05-10 |
| Intended Use | Continuous cardiac rhythm monitoring | Continuous cardiac rhythm monitoring |
| Measurement Accuracy | +-1 bpm | +-0.8 bpm |
| Data Storage | 30 days onboard memory | 60 days onboard memory |
| Wireless Connectivity | Bluetooth 4.0 | Bluetooth 5.0 |
| Battery Life | 48 hours | 72 hours |
| Dimensions | 5.5 x 3.5 x 1.2 cm | 5.7 x 3.7 x 1.3 cm |
| Weight | 45 g | 48 g |
| Safety Standards | IEC 60601-1, ISO 13485 | IEC 60601-1, ISO 13485 |
Introduction to Predicate and Reference Devices
Predicate devices serve as legally marketed medical devices used to demonstrate the substantial equivalence of a new device during the FDA 510(k) clearance process. Reference devices provide a baseline for comparison but may not have formal approval status, often used to support performance claims or benchmarking. Understanding the distinction between predicate and reference devices is crucial for regulatory submissions and product development in the medical device industry.
Regulatory Framework: Predicate vs Reference Devices
In the regulatory framework for medical devices, a predicate device is an already legally marketed device used by regulatory bodies like the FDA to determine substantial equivalence for 510(k) clearance. In contrast, a reference device serves as a benchmark for performance testing and scientific comparison, often used in clinical evaluations and research rather than direct regulatory submissions. Understanding the distinction between predicate and reference devices is crucial for navigating approval pathways and ensuring compliance with regulatory standards.
Definitions: Understanding Predicate Devices
A predicate device is a legally marketed medical device previously cleared by regulatory agencies such as the FDA, used as a baseline for evaluating new devices through the 510(k) premarket notification process. It serves as a benchmark by demonstrating that the new device is substantially equivalent in safety and effectiveness to an already approved product. Understanding predicate devices helps manufacturers navigate regulatory pathways and ensures that innovations meet established safety standards.
Definitions: Understanding Reference Devices
Reference devices in medical device regulation serve as a baseline for demonstrating substantial equivalence during the approval process, typically being legally marketed devices with similar intended use and technological characteristics. Unlike predicate devices, which are specifically used to establish safety and effectiveness for 510(k) submissions, reference devices provide a broader context for comparison, often cited in performance testing and clinical evaluation. Understanding the distinction between predicate and reference devices is crucial for manufacturers to navigate regulatory pathways efficiently and ensure compliance with standards.
Key Differences Between Predicate and Reference Devices
Predicate devices are legally marketed medical devices previously cleared by regulatory authorities, primarily used to demonstrate substantial equivalence during the FDA 510(k) submission process. Reference devices serve as benchmarks for performance testing and may not necessarily be cleared through the same regulatory pathway, focusing on technical comparison rather than legal clearance. Key differences include regulatory status, role in compliance evaluation, and specific use cases in device validation and approval workflows.
Impact on FDA 510(k) Submission
The distinction between a predicate device and a reference device plays a crucial role in FDA 510(k) submissions, where the predicate device serves as the primary benchmark demonstrating substantial equivalence for clearance. Reference devices provide supporting evidence but do not establish the foundational predicate comparison necessary for FDA review. Proper identification and comparison to a suitable predicate device can significantly streamline the FDA evaluation process and increase the likelihood of successful market authorization.
Selection Criteria for Predicate Devices
Selection criteria for predicate devices focus on substantial equivalence to the new medical device in terms of intended use, technological characteristics, and safety and effectiveness. The predicate device must have been legally marketed and share similar indications for use without significant differences that could affect performance or risk. Regulators evaluate these factors to ensure the new device maintains comparable safety and effectiveness standards.
When to Use a Reference Device
Use a reference device when the new medical device differs significantly in technology or intended use from existing predicate devices. Reference devices provide a benchmark for performance comparison when direct equivalence to a predicate device cannot be established. This approach ensures the new device meets safety and effectiveness standards through alternative validation methods.
Advantages and Limitations of Each Approach
Predicate devices enable streamlined FDA 510(k) clearance by demonstrating substantial equivalence to legally marketed devices, reducing time and costs in regulatory approval. Their limitation lies in potential propagation of outdated technologies and insufficient addressing of novel risks unique to the new device. Reference devices provide a benchmark for performance comparison, offering a broader scope for innovation assessment but may require more extensive clinical data, leading to longer development timelines and higher validation expenses.
Best Practices for Leveraging Predicate and Reference Devices
Leveraging predicate and reference devices effectively requires thorough comparison of technical characteristics, intended use, and performance data to ensure substantial equivalence and regulatory compliance. Best practices include comprehensive documentation of device similarities and differences, rigorous risk analysis, and alignment with FDA guidance to streamline the 510(k) submission process. Consistent communication with regulatory authorities and robust clinical evidence further enhance successful device clearance and market entry.
Predicate device vs Reference device Infographic
 productdif.com
productdif.com