Biocompatibility ensures that medical devices interact safely with biological tissues without causing adverse reactions, while biodegradability refers to the device's ability to break down within the body over time. Materials used in biocompatible devices must avoid toxicity and immune responses, whereas biodegradable materials are designed to degrade into non-toxic byproducts that the body can absorb or excrete. Selecting the appropriate balance between biocompatibility and biodegradability is critical for optimizing device performance and patient outcomes.
Table of Comparison
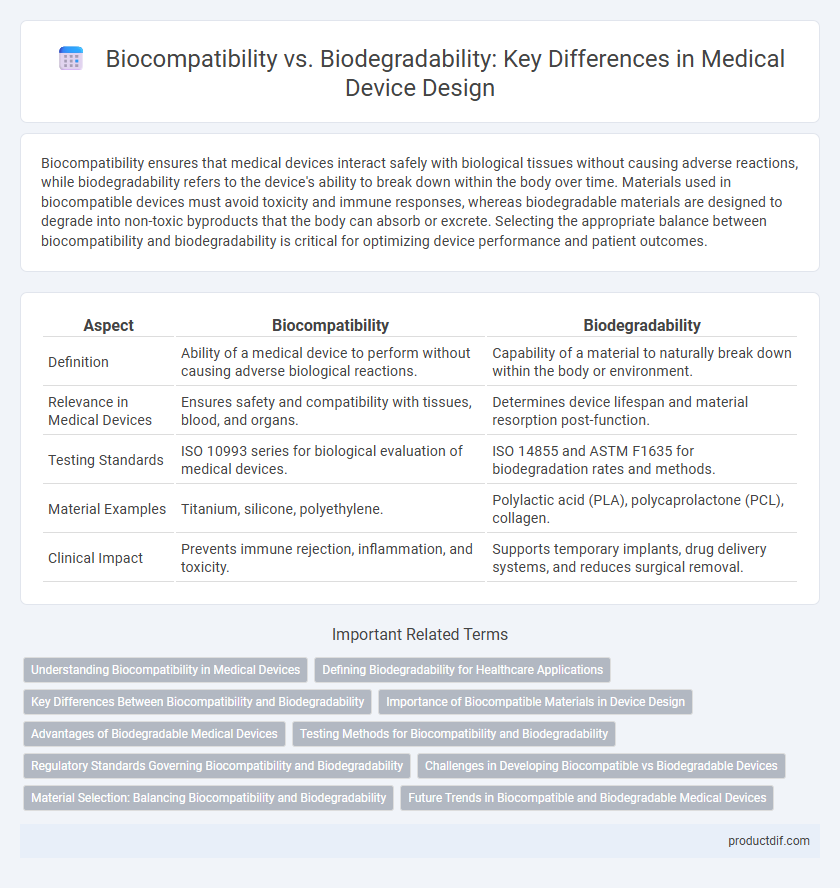
| Aspect | Biocompatibility | Biodegradability |
|---|---|---|
| Definition | Ability of a medical device to perform without causing adverse biological reactions. | Capability of a material to naturally break down within the body or environment. |
| Relevance in Medical Devices | Ensures safety and compatibility with tissues, blood, and organs. | Determines device lifespan and material resorption post-function. |
| Testing Standards | ISO 10993 series for biological evaluation of medical devices. | ISO 14855 and ASTM F1635 for biodegradation rates and methods. |
| Material Examples | Titanium, silicone, polyethylene. | Polylactic acid (PLA), polycaprolactone (PCL), collagen. |
| Clinical Impact | Prevents immune rejection, inflammation, and toxicity. | Supports temporary implants, drug delivery systems, and reduces surgical removal. |
Understanding Biocompatibility in Medical Devices
Biocompatibility in medical devices ensures that materials do not elicit adverse biological responses when implanted or in contact with body tissues, crucial for patient safety and device efficacy. Unlike biodegradability, which refers to a material's ability to break down naturally over time, biocompatibility emphasizes the interaction between the device and the host environment without causing toxicity, inflammation, or immune rejection. Standards such as ISO 10993 guide the evaluation of biocompatibility, encompassing cytotoxicity, sensitization, and systemic toxicity tests to validate device safety before clinical use.
Defining Biodegradability for Healthcare Applications
Biodegradability in healthcare applications refers to the ability of a medical device material to break down safely within the human body through natural biological processes, minimizing long-term environmental impact and reducing the need for surgical removal. It is essential to differentiate biodegradability from biocompatibility, which assesses material compatibility with body tissues without causing adverse reactions. Defining biodegradability involves evaluating the rate and byproducts of degradation to ensure they are non-toxic and promote patient safety in implantable and absorbable medical devices.
Key Differences Between Biocompatibility and Biodegradability
Biocompatibility refers to a medical device's ability to perform safely and effectively in contact with the human body without causing adverse reactions, while biodegradability denotes the capability of the device material to naturally break down and be absorbed or eliminated by biological processes. Key differences include that biocompatibility emphasizes non-toxicity and minimal immune response, whereas biodegradability focuses on material decomposition and environmental compatibility. These distinct properties influence device design choices, regulatory approvals, and clinical applications based on patient safety and device lifecycle requirements.
Importance of Biocompatible Materials in Device Design
Biocompatible materials play a crucial role in medical device design by ensuring that implants and instruments do not trigger adverse immune responses, thereby promoting patient safety and device efficacy. Unlike biodegradable materials, which are engineered to break down within the body over time, biocompatible materials must maintain stability and functionality without causing toxicity or inflammation. Prioritizing biocompatibility enhances the success rate of medical devices in applications such as prosthetics, cardiovascular stents, and orthopedic implants.
Advantages of Biodegradable Medical Devices
Biodegradable medical devices offer significant advantages by eliminating the need for surgical removal, reducing patient risk and healthcare costs. These devices naturally break down into non-toxic byproducts, minimizing long-term foreign body reactions and promoting tissue regeneration. Their use enhances patient recovery and supports sustainable medical practices through reduced medical waste.
Testing Methods for Biocompatibility and Biodegradability
Biocompatibility testing methods for medical devices emphasize cytotoxicity, sensitization, and irritation assessments following ISO 10993 standards to ensure safe tissue interaction. Biodegradability testing involves evaluating material degradation through in vitro assays like enzymatic hydrolysis and in vivo implantation studies measuring molecular weight changes and mass loss over time. Combining these testing protocols is essential to validate medical device materials that are both biologically safe and capable of predictable degradation within the human body.
Regulatory Standards Governing Biocompatibility and Biodegradability
Regulatory standards for medical devices require rigorous evaluation of biocompatibility according to ISO 10993 series, ensuring materials do not produce adverse biological reactions in patients. Biodegradability standards focus on controlled degradation rates and byproducts, following guidelines from FDA and ASTM to confirm safety and efficacy over time. Compliance with these frameworks ensures that devices meet both biological safety and functional longevity for clinical applications.
Challenges in Developing Biocompatible vs Biodegradable Devices
Developing biocompatible medical devices requires overcoming immune response challenges and ensuring long-term tissue integration without toxicity. Biodegradable devices face the complexity of controlling degradation rates while maintaining mechanical stability and avoiding harmful byproducts. Balancing these factors demands advanced material science and rigorous in vivo testing to optimize safety and functionality.
Material Selection: Balancing Biocompatibility and Biodegradability
Selecting materials for medical devices requires a precise balance between biocompatibility and biodegradability to ensure both patient safety and device functionality. Biocompatible materials minimize adverse immune responses, while biodegradable components enable controlled degradation within the body, reducing the need for surgical removal. Optimal material selection integrates polymers like polylactic acid (PLA) or polycaprolactone (PCL) that demonstrate favorable degradation profiles alongside minimal cytotoxicity in clinical applications.
Future Trends in Biocompatible and Biodegradable Medical Devices
Future trends in biocompatible and biodegradable medical devices emphasize the integration of advanced biomaterials that enhance tissue regeneration while minimizing immune response and toxicity. Innovations in polymer science and nanotechnology enable the development of devices that safely degrade within the body, reducing the need for surgical removal and improving patient outcomes. Research increasingly focuses on multifunctional materials combining biocompatibility with controlled biodegradability tailored to specific clinical applications such as drug delivery and implantable sensors.
Biocompatibility vs Biodegradability Infographic
 productdif.com
productdif.com