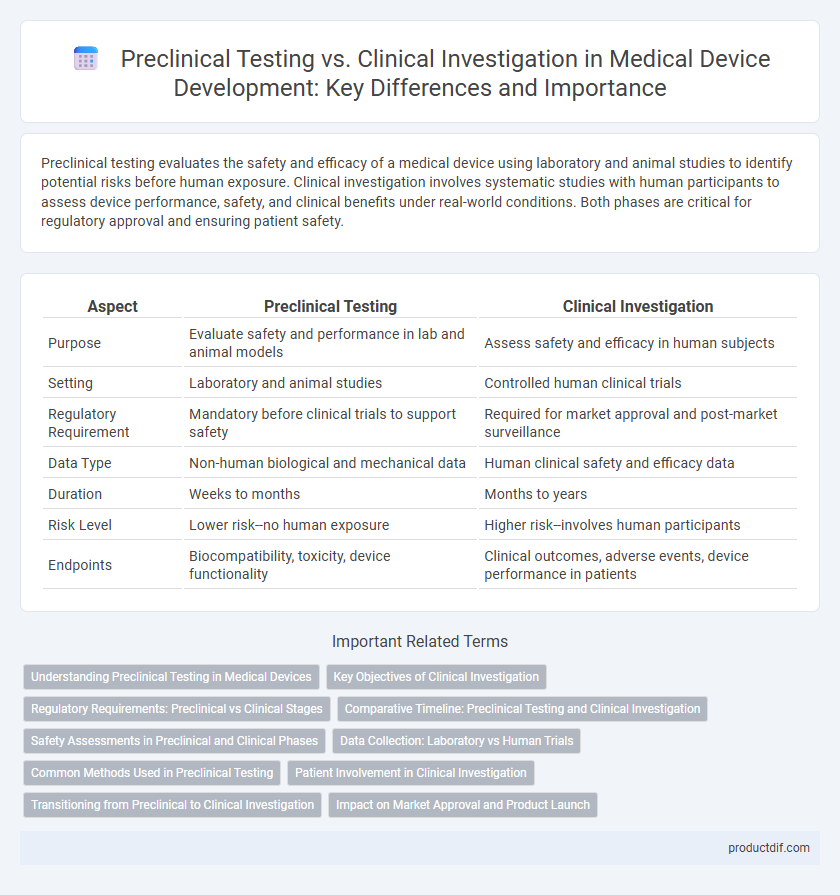
Preclinical testing evaluates the safety and efficacy of a medical device using laboratory and animal studies to identify potential risks before human exposure. Clinical investigation involves systematic studies with human participants to assess device performance, safety, and clinical benefits under real-world conditions. Both phases are critical for regulatory approval and ensuring patient safety.
Table of Comparison
| Aspect | Preclinical Testing | Clinical Investigation |
|---|---|---|
| Purpose | Evaluate safety and performance in lab and animal models | Assess safety and efficacy in human subjects |
| Setting | Laboratory and animal studies | Controlled human clinical trials |
| Regulatory Requirement | Mandatory before clinical trials to support safety | Required for market approval and post-market surveillance |
| Data Type | Non-human biological and mechanical data | Human clinical safety and efficacy data |
| Duration | Weeks to months | Months to years |
| Risk Level | Lower risk--no human exposure | Higher risk--involves human participants |
| Endpoints | Biocompatibility, toxicity, device functionality | Clinical outcomes, adverse events, device performance in patients |
Understanding Preclinical Testing in Medical Devices
Preclinical testing in medical devices involves rigorous laboratory and animal studies designed to evaluate the safety, functionality, and biocompatibility of the device before human trials. This phase ensures the device meets regulatory standards and identifies potential risks, thereby minimizing hazards during subsequent clinical investigations. Understanding these tests is crucial to optimizing device design and supporting successful regulatory approval.
Key Objectives of Clinical Investigation
Clinical investigation primarily aims to evaluate the safety and effectiveness of medical devices in humans by generating robust clinical evidence. It focuses on assessing device performance, identifying potential risks, and confirming benefits under real-world conditions to support regulatory approval. Preclinical testing, in contrast, involves laboratory and animal studies designed to establish initial safety and functionality before human trials commence.
Regulatory Requirements: Preclinical vs Clinical Stages
Preclinical testing involves comprehensive laboratory and animal studies to assess the safety, biocompatibility, and functionality of medical devices before human exposure, meeting regulatory standards like ISO 10993 and FDA's Good Laboratory Practice (GLP) requirements. Clinical investigation follows preclinical approval, focusing on evaluating the device's safety and performance in human subjects under protocols approved by regulatory bodies such as the FDA (21 CFR Part 812) and the European MDR, ensuring compliance with Good Clinical Practice (GCP). Regulatory requirements emphasize rigorous documentation, risk analysis, and ethical considerations at both stages to support device approval and market entry.
Comparative Timeline: Preclinical Testing and Clinical Investigation
Preclinical testing for medical devices typically spans 6 to 18 months, encompassing in vitro and in vivo studies to evaluate safety and functionality before human exposure. Clinical investigation follows, lasting 1 to 3 years, where the device is tested in controlled human trials to assess efficacy, safety, and performance under real-world conditions. The combined timeline ensures thorough evaluation, aligning regulatory requirements with patient safety and effective clinical outcomes.
Safety Assessments in Preclinical and Clinical Phases
Preclinical testing evaluates medical device safety through in vitro and in vivo studies, identifying potential toxicological, biocompatibility, and mechanical risks before human use. Clinical investigation further assesses device safety by monitoring adverse events, performance outcomes, and patient tolerability in controlled human trials. Rigorous safety assessments in both phases ensure regulatory compliance and minimize risks throughout the device development lifecycle.
Data Collection: Laboratory vs Human Trials
Preclinical testing primarily involves data collection through laboratory experiments, including in vitro and in vivo studies, to evaluate the safety, biocompatibility, and functionality of medical devices before human exposure. Clinical investigation collects data from human trials to assess device performance, efficacy, and adverse effects in real-world medical scenarios, providing critical evidence for regulatory approval. The transition from controlled laboratory data to clinical data ensures comprehensive validation, minimizing risks and optimizing patient outcomes.
Common Methods Used in Preclinical Testing
Preclinical testing in medical device development primarily involves in vitro studies, animal testing, and biomechanical assessments to evaluate safety, functionality, and biocompatibility before human trials. These methods help identify potential risks and device performance issues by simulating physiological conditions and interactions with biological tissues. Data obtained from preclinical testing establish a foundation for regulatory submissions and guide the design of clinical investigations.
Patient Involvement in Clinical Investigation
Patient involvement in clinical investigation is critical for assessing the safety, efficacy, and usability of medical devices in real-world settings, providing vital data that preclinical testing cannot capture. Unlike preclinical testing, which utilizes laboratory and animal models to evaluate device function and biocompatibility, clinical investigation directly involves human subjects to identify potential risks, adverse events, and therapeutic benefits. Regulatory agencies require robust clinical data with active patient participation to support device approval and market clearance, ensuring that medical devices meet patient needs and clinical performance standards.
Transitioning from Preclinical to Clinical Investigation
Transitioning from preclinical testing to clinical investigation involves rigorous evaluation of safety and efficacy data derived from laboratory and animal studies to meet regulatory requirements. Preclinical testing focuses on biological compatibility, toxicology, and device functionality, establishing a foundation for initiating first-in-human trials. Ensuring comprehensive preclinical data supports clinical trial design, risk mitigation, and patient safety during the clinical phases of medical device development.
Impact on Market Approval and Product Launch
Preclinical testing provides essential safety and performance data through in vitro and animal studies, forming the regulatory foundation required for clinical investigation authorization. Clinical investigation generates critical human trial evidence demonstrating device efficacy and safety, directly influencing regulatory approval decisions and market entry timelines. Robust data from both phases accelerate regulatory clearance, reduce post-market risks, and enable timely product launch strategies.
Preclinical testing vs Clinical investigation Infographic
 productdif.com
productdif.com