A Quality Management System (QMS) in medical devices ensures consistent product quality and regulatory compliance through systematic processes and documentation. In contrast, a Risk Management System (RMS) focuses on identifying, assessing, and mitigating potential hazards to patient safety throughout the product lifecycle. Integrating QMS with RMS enhances overall device reliability and reduces the likelihood of adverse events.
Table of Comparison
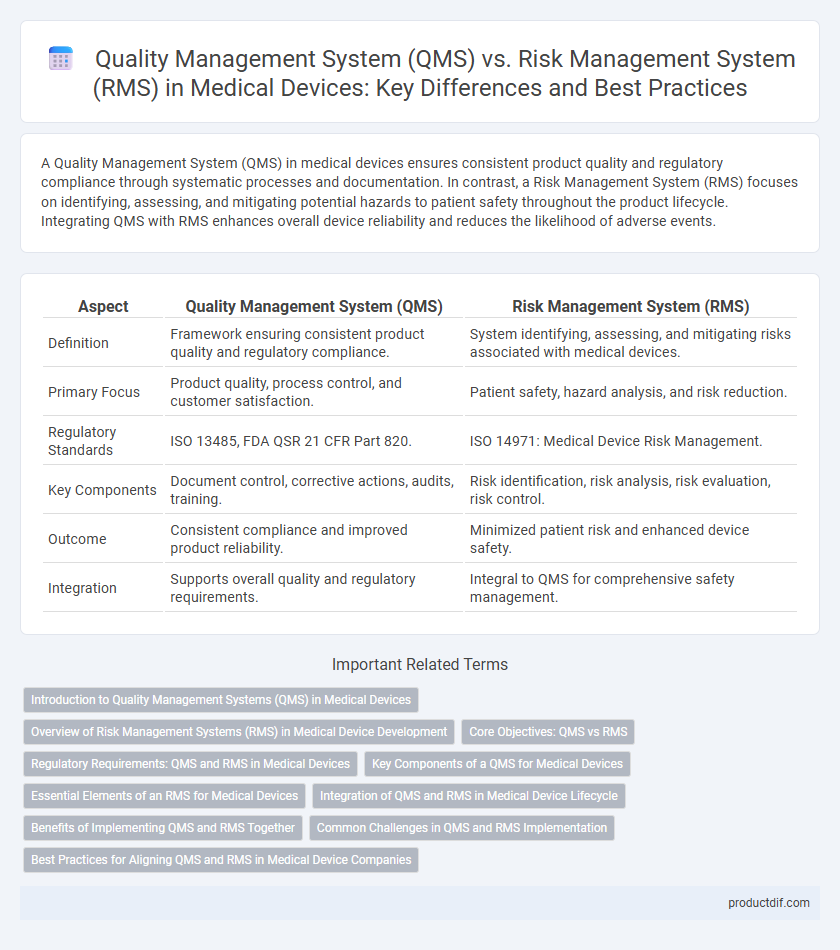
| Aspect | Quality Management System (QMS) | Risk Management System (RMS) |
|---|---|---|
| Definition | Framework ensuring consistent product quality and regulatory compliance. | System identifying, assessing, and mitigating risks associated with medical devices. |
| Primary Focus | Product quality, process control, and customer satisfaction. | Patient safety, hazard analysis, and risk reduction. |
| Regulatory Standards | ISO 13485, FDA QSR 21 CFR Part 820. | ISO 14971: Medical Device Risk Management. |
| Key Components | Document control, corrective actions, audits, training. | Risk identification, risk analysis, risk evaluation, risk control. |
| Outcome | Consistent compliance and improved product reliability. | Minimized patient risk and enhanced device safety. |
| Integration | Supports overall quality and regulatory requirements. | Integral to QMS for comprehensive safety management. |
Introduction to Quality Management Systems (QMS) in Medical Devices
Quality Management Systems (QMS) in medical devices are structured frameworks designed to ensure product safety, efficacy, and regulatory compliance throughout the device lifecycle. QMS integrates processes such as design control, production, and post-market surveillance to systematically manage quality risks and improve device reliability. Implementing a robust QMS aligns with international standards like ISO 13485, facilitating consistent quality output and enhancing patient safety.
Overview of Risk Management Systems (RMS) in Medical Device Development
Risk Management Systems (RMS) in medical device development systematically identify, assess, and mitigate potential hazards to ensure patient safety and regulatory compliance. RMS integrates processes such as ISO 14971 standards for risk analysis, evaluation, control, and post-market surveillance to maintain device reliability throughout its lifecycle. Effective RMS implementation directly supports Quality Management Systems (QMS) by providing critical data for continuous improvement and reducing product-related risks.
Core Objectives: QMS vs RMS
The core objective of a Quality Management System (QMS) in medical devices is to ensure consistent product quality and regulatory compliance through structured processes and continuous improvement. In contrast, the Risk Management System (RMS) focuses on identifying, evaluating, and mitigating potential hazards associated with the medical device to enhance patient safety and minimize risks. Both systems are integral, with QMS driving overall process control and RMS targeting specific safety concerns throughout the device lifecycle.
Regulatory Requirements: QMS and RMS in Medical Devices
Regulatory requirements for medical devices mandate the integration of both Quality Management Systems (QMS) and Risk Management Systems (RMS) to ensure safety and compliance. QMS standards like ISO 13485 emphasize consistent manufacturing processes and documentation controls, while RMS follows ISO 14971 guidelines to identify, evaluate, and mitigate potential device risks throughout the product lifecycle. Regulatory bodies such as the FDA and MDR require thorough risk analysis linked with quality processes to meet market approval and post-market surveillance obligations.
Key Components of a QMS for Medical Devices
Key components of a Quality Management System (QMS) for medical devices include document control, design controls, corrective and preventive actions (CAPA), and supplier management. These elements ensure regulatory compliance, continuous product improvement, and patient safety throughout the device lifecycle. Integration with risk management processes supports hazard identification, risk analysis, and mitigation strategies essential for meeting ISO 13485 and FDA 21 CFR Part 820 standards.
Essential Elements of an RMS for Medical Devices
An effective Risk Management System (RMS) for medical devices must include comprehensive hazard identification, risk analysis, risk evaluation, and risk control processes to ensure patient safety and device efficacy. Key elements involve continuous monitoring and feedback mechanisms for post-market surveillance, documentation of residual risks, and clear communication across cross-functional teams to comply with ISO 14971 standards. Integration with the Quality Management System (QMS) supports compliance with regulatory requirements such as FDA 21 CFR Part 820 and MDR, ensuring systematic risk mitigation throughout the product lifecycle.
Integration of QMS and RMS in Medical Device Lifecycle
Integrating Quality Management System (QMS) and Risk Management System (RMS) in the medical device lifecycle enhances compliance with ISO 13485 and ISO 14971 standards by ensuring continuous monitoring and mitigation of risks alongside quality assurance processes. This integration facilitates traceability from risk assessment to corrective actions, improving product safety and regulatory submissions. Efficient synchronization of QMS and RMS accelerates decision-making, reduces nonconformities, and supports post-market surveillance activities throughout the device lifecycle.
Benefits of Implementing QMS and RMS Together
Implementing both Quality Management System (QMS) and Risk Management System (RMS) in medical device manufacturing enhances product safety and regulatory compliance through systematic quality control and proactive risk identification. The integration ensures continuous improvement by aligning quality processes with risk mitigation strategies, reducing product recalls and liability exposure. Combined QMS and RMS foster a culture of accountability and efficiency, driving innovation while meeting ISO 13485 and ISO 14971 standards.
Common Challenges in QMS and RMS Implementation
Common challenges in implementing Quality Management Systems (QMS) and Risk Management Systems (RMS) in medical device manufacturing include inadequate integration between processes, leading to inefficiencies and compliance gaps. Deficiencies in employee training and awareness often result in inconsistent application of standardized procedures and risk controls. Maintaining comprehensive documentation that aligns with regulatory standards such as ISO 13485 and ISO 14971 poses ongoing challenges for both QMS and RMS effectiveness.
Best Practices for Aligning QMS and RMS in Medical Device Companies
Aligning Quality Management System (QMS) and Risk Management System (RMS) in medical device companies enhances regulatory compliance and product safety by integrating risk analysis into all quality processes. Best practices include establishing cross-functional teams to ensure seamless communication, implementing unified documentation protocols, and utilizing risk-based decision-making tools within the QMS framework. Leveraging standards such as ISO 13485 for QMS and ISO 14971 for RMS promotes consistency and continuous improvement across product lifecycle management.
Quality Management System (QMS) vs Risk Management System (RMS) Infographic
 productdif.com
productdif.com