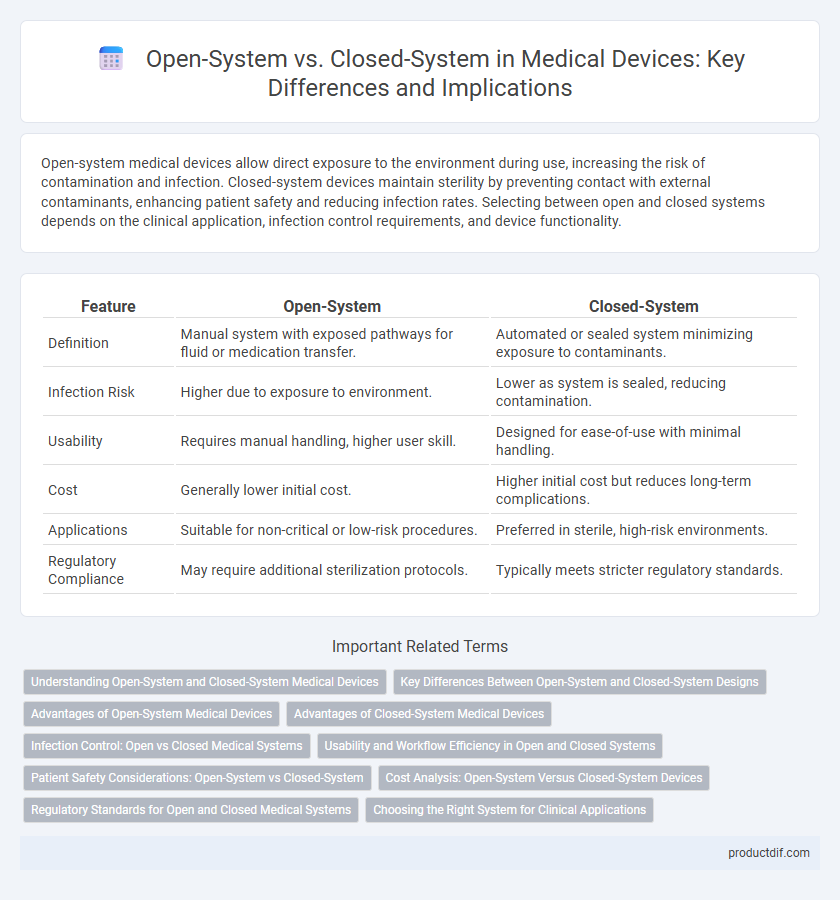
Open-system medical devices allow direct exposure to the environment during use, increasing the risk of contamination and infection. Closed-system devices maintain sterility by preventing contact with external contaminants, enhancing patient safety and reducing infection rates. Selecting between open and closed systems depends on the clinical application, infection control requirements, and device functionality.
Table of Comparison
| Feature | Open-System | Closed-System |
|---|---|---|
| Definition | Manual system with exposed pathways for fluid or medication transfer. | Automated or sealed system minimizing exposure to contaminants. |
| Infection Risk | Higher due to exposure to environment. | Lower as system is sealed, reducing contamination. |
| Usability | Requires manual handling, higher user skill. | Designed for ease-of-use with minimal handling. |
| Cost | Generally lower initial cost. | Higher initial cost but reduces long-term complications. |
| Applications | Suitable for non-critical or low-risk procedures. | Preferred in sterile, high-risk environments. |
| Regulatory Compliance | May require additional sterilization protocols. | Typically meets stricter regulatory standards. |
Understanding Open-System and Closed-System Medical Devices
Open-system medical devices allow components or fluids to interact with the external environment, increasing flexibility but also posing higher contamination risks. Closed-system medical devices maintain a sealed environment to prevent exposure, ensuring sterility and reducing infection chances during use. Understanding these systems is crucial for selecting appropriate devices based on safety requirements and clinical applications.
Key Differences Between Open-System and Closed-System Designs
Open-system medical devices allow direct interaction with external environments, enabling easy maintenance and customization but increasing contamination risks. Closed-system designs feature sealed units that prevent external exposure, enhancing sterility and safety but limiting flexibility and requiring specialized servicing. Key differences include contamination control, user accessibility, and design complexity, which impact device application and regulatory compliance.
Advantages of Open-System Medical Devices
Open-system medical devices offer enhanced flexibility by allowing seamless integration with multiple third-party software and hardware, facilitating customized workflows tailored to specific clinical needs. These devices support real-time data sharing and interoperability across diverse healthcare platforms, improving patient monitoring and decision-making efficiency. Open systems also promote cost-effectiveness by enabling incremental upgrades and reducing dependency on proprietary technologies, leading to better resource management in healthcare settings.
Advantages of Closed-System Medical Devices
Closed-system medical devices reduce the risk of contamination and infection by preventing exposure to environmental pathogens, ensuring enhanced patient safety. They offer improved control over dosing accuracy and minimize medication errors through integrated design features. These devices also facilitate easier compliance with regulatory standards by maintaining sterility throughout the procedure.
Infection Control: Open vs Closed Medical Systems
Closed medical systems significantly reduce the risk of infection by preventing microbial contamination during fluid transfer or medication administration, unlike open systems that expose internal components to the environment. Utilizing closed-system technology minimizes the potential for pathogen entry, thereby enhancing patient safety and compliance with infection control standards. Hospitals and healthcare facilities widely adopt closed systems to maintain sterility and reduce healthcare-associated infections (HAIs).
Usability and Workflow Efficiency in Open and Closed Systems
Open systems in medical devices offer greater flexibility by allowing interoperability with multiple platforms, which enhances workflow efficiency by enabling seamless integration and customization. Closed systems prioritize user safety and standardization through a controlled environment, reducing the risk of errors but potentially limiting usability due to restricted compatibility. Efficient usability in open systems supports dynamic clinical environments, whereas closed systems optimize standardized procedures with consistent performance.
Patient Safety Considerations: Open-System vs Closed-System
Closed-system medical devices minimize patient exposure to contaminants by preventing external environmental contact, significantly reducing infection risks during procedures. Open-system devices, while offering ease of access and flexibility, pose higher risks of microbial contamination and cross-infection, necessitating stringent sterilization protocols. Choosing closed-system technology enhances patient safety by maintaining aseptic conditions and reducing the incidence of healthcare-associated infections (HAIs).
Cost Analysis: Open-System Versus Closed-System Devices
Open-system medical devices often incur higher long-term costs due to increased risk of contamination and maintenance requirements, despite lower upfront expenses. Closed-system devices typically present higher initial investment but reduce operational costs through improved sterility, decreased risk of infection, and simplified handling. Comprehensive cost analysis reveals that closed-system technology can offer better value over time by minimizing patient complications and regulatory compliance issues.
Regulatory Standards for Open and Closed Medical Systems
Open medical systems require stringent compliance with interoperability and data exchange standards such as HL7 and IEEE 11073 to ensure patient safety and device functionality. Closed medical systems must adhere to comprehensive regulatory frameworks like ISO 13485 and FDA 21 CFR Part 820, emphasizing device security, manufacturing controls, and risk management. Regulatory agencies impose specific validation and documentation protocols to guarantee both open and closed systems maintain consistent performance and minimize contamination risks in clinical environments.
Choosing the Right System for Clinical Applications
Selecting the appropriate medical device system depends on the specific clinical application requirements, with open-systems offering flexibility and customization for complex procedures, while closed-systems provide enhanced sterility and reduced contamination risk. Closed-systems are preferred in environments demanding strict infection control, such as chemotherapy drug preparation or blood transfusions, due to their airtight design preventing exposure to pathogens. Open-systems may be advantageous in diagnostics or therapeutic settings where adaptability and easy access to components are critical for tailored patient care.
Open-system vs Closed-system Infographic
 productdif.com
productdif.com