IVD devices are specialized medical tools designed for in vitro diagnostics, analyzing biological samples like blood or tissue to detect diseases and conditions. General medical devices encompass a broad range of instruments used directly on or inside the body for treatment, monitoring, or surgery. Unlike general medical devices, IVD devices require specific regulatory compliance focused on laboratory accuracy and patient safety in diagnostic results.
Table of Comparison
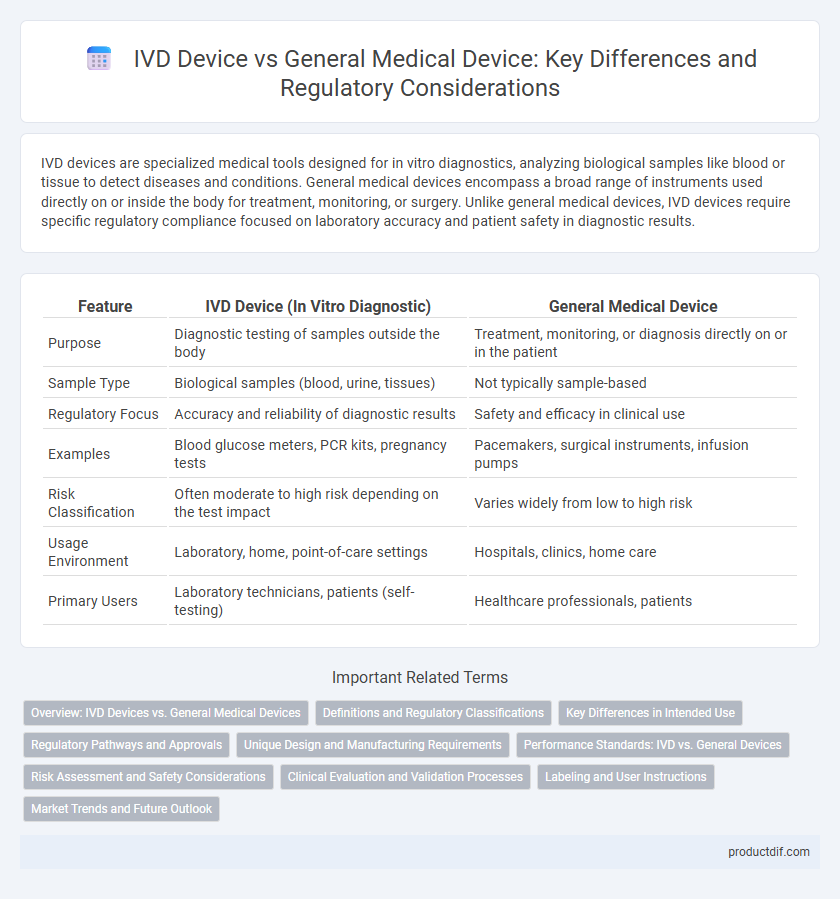
| Feature | IVD Device (In Vitro Diagnostic) | General Medical Device |
|---|---|---|
| Purpose | Diagnostic testing of samples outside the body | Treatment, monitoring, or diagnosis directly on or in the patient |
| Sample Type | Biological samples (blood, urine, tissues) | Not typically sample-based |
| Regulatory Focus | Accuracy and reliability of diagnostic results | Safety and efficacy in clinical use |
| Examples | Blood glucose meters, PCR kits, pregnancy tests | Pacemakers, surgical instruments, infusion pumps |
| Risk Classification | Often moderate to high risk depending on the test impact | Varies widely from low to high risk |
| Usage Environment | Laboratory, home, point-of-care settings | Hospitals, clinics, home care |
| Primary Users | Laboratory technicians, patients (self-testing) | Healthcare professionals, patients |
Overview: IVD Devices vs. General Medical Devices
IVD devices, or in vitro diagnostic devices, are specifically designed to analyze samples such as blood, tissue, or urine to provide crucial information for diagnosing diseases or conditions, whereas general medical devices encompass a broader range of products used for treatment, monitoring, or support of patients. IVD devices are subject to stringent regulatory requirements focusing on accuracy, reliability, and clinical validity due to their role in diagnosis, often classified under specific risk categories based on their intended use. In contrast, general medical devices vary widely in complexity and function, with regulatory controls tailored to their varying levels of risk and usage environments.
Definitions and Regulatory Classifications
In vitro diagnostic (IVD) devices are medical devices specifically designed to perform tests on samples taken from the human body, such as blood, tissue, or urine, to detect diseases, conditions, or infections. General medical devices encompass a broader range of instruments, apparatuses, or machines used for medical purposes without involving analysis of biological samples. Regulatory classifications for IVD devices are distinct and typically more stringent due to the direct impact on patient diagnosis and treatment, with categories often including self-testing, companion diagnostics, and routine testing devices, whereas general medical devices are classified based on risk levels ranging from Class I (low risk) to Class III (high risk) under frameworks like the FDA and MDR.
Key Differences in Intended Use
In vitro diagnostic (IVD) devices are specifically designed for the analysis of human samples such as blood or tissue to provide critical information for disease diagnosis, prognosis, or treatment monitoring, unlike general medical devices which are intended primarily for therapeutic, monitoring, or supportive purposes directly applied to patients. IVD devices require specialized regulatory pathways emphasizing accuracy, reliability, and clinical performance due to their direct impact on clinical decision-making. General medical devices encompass a broad range of equipment, including surgical instruments and implantable devices, with intended uses centered on physical intervention or support rather than biochemical analysis.
Regulatory Pathways and Approvals
In vitro diagnostic (IVD) devices undergo distinct regulatory pathways compared to general medical devices, typically requiring compliance with specific standards such as ISO 13485 and specialized evaluation of analytical and clinical performance data. Regulatory approvals for IVDs often involve stringent reviews by agencies like the FDA's Center for Devices and Radiological Health (CDRH) or the European Medicines Agency (EMA) under the In Vitro Diagnostic Regulation (IVDR), emphasizing accuracy, reliability, and patient safety. Conversely, general medical devices follow broader regulatory frameworks, including the FDA's premarket notification (510(k)) or premarket approval (PMA) processes based on device classification and risk level.
Unique Design and Manufacturing Requirements
IVD devices require unique design considerations to ensure accuracy and reliability in diagnosing diseases from biological samples, incorporating specialized reagents and detection mechanisms. Manufacturing processes for IVDs demand stringent contamination controls and validation protocols to maintain sensitivity and specificity. General medical devices, while also subject to rigorous standards, focus more on mechanical integrity and biocompatibility without the extensive biochemical interaction constraints seen in IVD production.
Performance Standards: IVD vs. General Devices
IVD devices require specialized performance standards focused on analytical accuracy, sensitivity, specificity, and reproducibility to ensure reliable diagnostic results from patient samples. General medical devices adhere to broader performance criteria, emphasizing safety, functionality, durability, and user interface without necessarily addressing diagnostic accuracy. Regulatory frameworks such as ISO 15197 for glucose monitoring and CLSI guidelines define rigorous performance standards specifically tailored for IVDs, differentiating them from general medical device standards like ISO 13485.
Risk Assessment and Safety Considerations
In vitro diagnostic (IVD) devices require rigorous risk assessment protocols due to their direct impact on clinical decisions and patient outcomes, emphasizing accuracy, sensitivity, and specificity. General medical devices focus risk assessments on mechanical safety, biocompatibility, and functional reliability during use. Safety considerations for IVDs prioritize contamination control and sample integrity, while general devices address physical hazards and device failure modes.
Clinical Evaluation and Validation Processes
IVD devices require rigorous clinical evaluation focusing on analytical and clinical performance to ensure accurate diagnostic results, while general medical devices emphasize safety and effectiveness for therapeutic or monitoring purposes. Validation processes for IVDs involve verifying test accuracy, reproducibility, and clinical utility with patient samples, contrasting with general devices where validation centers on functional performance and biocompatibility. Regulatory standards such as ISO 13485 and guidelines from agencies like the FDA and EMA dictate specific clinical evaluation protocols tailored to each device category.
Labeling and User Instructions
Labeling for in vitro diagnostic (IVD) devices must comply with specific regulatory standards such as ISO 15189 and include detailed information about specimen type, test limitations, and diagnostic indications to ensure accurate interpretation. User instructions for IVD devices require clear guidance on sample collection, handling, and processing protocols, often incorporating diagrams or step-by-step procedures tailored to laboratory or point-of-care settings. General medical device labeling emphasizes safety warnings, device specifications, and maintenance instructions but typically involves less detailed diagnostic or specimen-related information compared to IVD devices.
Market Trends and Future Outlook
The In Vitro Diagnostic (IVD) device market is experiencing rapid growth driven by advances in molecular diagnostics, point-of-care testing, and personalized medicine, outperforming the broader general medical device sector. Increasing demand for early disease detection and regulatory support is fueling innovation and adoption of IVD technologies worldwide. Future outlook predicts sustained expansion with integration of AI and IoT, positioning IVD devices as critical tools in precision healthcare and pandemic preparedness.
IVD device vs General medical device Infographic
 productdif.com
productdif.com