Sterile packaging ensures medical devices remain free from microorganisms, reducing infection risks during procedures. Non-sterile packaging is suitable for devices that undergo sterilization before use or for non-invasive applications. Choosing between sterile and non-sterile packaging depends on the device's intended use and regulatory requirements.
Table of Comparison
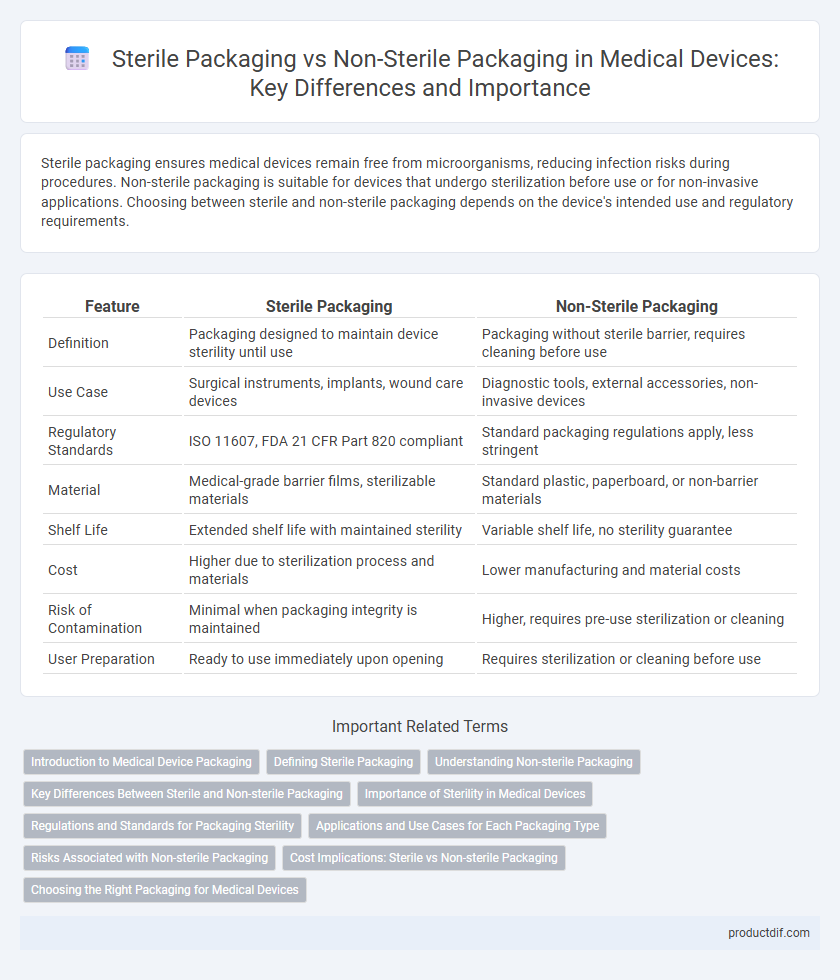
| Feature | Sterile Packaging | Non-Sterile Packaging |
|---|---|---|
| Definition | Packaging designed to maintain device sterility until use | Packaging without sterile barrier, requires cleaning before use |
| Use Case | Surgical instruments, implants, wound care devices | Diagnostic tools, external accessories, non-invasive devices |
| Regulatory Standards | ISO 11607, FDA 21 CFR Part 820 compliant | Standard packaging regulations apply, less stringent |
| Material | Medical-grade barrier films, sterilizable materials | Standard plastic, paperboard, or non-barrier materials |
| Shelf Life | Extended shelf life with maintained sterility | Variable shelf life, no sterility guarantee |
| Cost | Higher due to sterilization process and materials | Lower manufacturing and material costs |
| Risk of Contamination | Minimal when packaging integrity is maintained | Higher, requires pre-use sterilization or cleaning |
| User Preparation | Ready to use immediately upon opening | Requires sterilization or cleaning before use |
Introduction to Medical Device Packaging
Sterile packaging ensures medical devices remain free from contaminants, maintaining aseptic conditions crucial for surgical tools and implants, whereas non-sterile packaging is suitable for devices used in non-invasive or diagnostic settings. The choice between sterile and non-sterile packaging directly impacts device shelf life, user safety, and regulatory compliance, with sterile packaging often involving complex materials and sealing techniques like Tyvek pouches or rigid trays. Effective medical device packaging incorporates barrier properties, tamper evidence, and biocompatibility to preserve device integrity and support hospital or clinical workflows.
Defining Sterile Packaging
Sterile packaging in medical devices ensures that the product inside remains free from viable microorganisms until the point of use, meeting stringent regulatory standards such as ISO 11607. This packaging employs materials and sealing techniques designed to maintain sterility through transportation and storage, often incorporating barrier properties against contaminants. Unlike non-sterile packaging, sterile packaging undergoes validated sterilization processes like gamma irradiation or ethylene oxide to guarantee microbial safety for surgical and diagnostic applications.
Understanding Non-sterile Packaging
Non-sterile packaging in medical devices provides protection against contamination during storage and transportation but does not guarantee a sterile product upon opening. It is commonly used for devices that can be sterilized prior to use or are intended for non-critical applications where sterility is not mandatory. Understanding the specifications and limitations of non-sterile packaging is essential for compliance with regulatory standards such as ISO 11607 and for ensuring patient safety.
Key Differences Between Sterile and Non-sterile Packaging
Sterile packaging ensures medical devices remain free from viable microorganisms, maintaining aseptic conditions critical for surgical and invasive procedures, while non-sterile packaging does not guarantee microbial elimination and is suitable for devices intended for non-invasive use. Sterile packaging often involves materials like Tyvek or medical-grade plastic films designed to withstand sterilization processes such as gamma irradiation or ethylene oxide gas, ensuring microbial barrier integrity. In contrast, non-sterile packaging prioritizes basic protection from physical damage and contamination without sterilization validation, impacting device handling, regulatory requirements, and clinical application.
Importance of Sterility in Medical Devices
Sterile packaging in medical devices is critical to preventing microbial contamination and ensuring patient safety during invasive procedures. Maintaining sterility reduces the risk of infections, which can lead to severe complications and extended hospital stays. Non-sterile packaging is suitable only for devices that do not enter sterile body sites, highlighting the importance of selecting appropriate packaging to meet regulatory standards and clinical requirements.
Regulations and Standards for Packaging Sterility
Medical device sterile packaging must comply with ISO 11607, which sets requirements for materials, design, and validation to maintain sterility until point of use. Non-sterile packaging is subject to general packaging standards such as ASTM D4169 but does not require sterility assurance levels or bioburden control. Regulatory bodies like the FDA enforce strict validation protocols for sterile packaging, including integrity testing, microbial barrier performance, and documentation to ensure patient safety.
Applications and Use Cases for Each Packaging Type
Sterile packaging is crucial for medical devices used in surgical procedures, wound care, and implantable products, ensuring they remain free from contaminants to prevent infections. Non-sterile packaging is suitable for devices used in non-invasive diagnostics, rehabilitation equipment, and single-use consumables where sterility is not critical. The choice between sterile and non-sterile packaging depends on the device's intended use, regulatory requirements, and risk of contamination during patient contact.
Risks Associated with Non-sterile Packaging
Non-sterile packaging of medical devices poses significant risks including contamination by bacteria, viruses, and other pathogens, which can lead to serious infections in patients. The lack of a sterile barrier increases the likelihood of microbial ingress during storage, transport, and handling, compromising device safety and efficacy. Regulatory bodies such as the FDA and ISO emphasize stringent sterile packaging standards to minimize infection risks and ensure patient safety.
Cost Implications: Sterile vs Non-sterile Packaging
Sterile packaging for medical devices typically incurs higher costs due to the need for meticulous sterilization processes, specialized materials, and compliance with stringent regulatory standards. Non-sterile packaging, while more cost-effective, requires additional sterilization steps before clinical use, potentially increasing overall operational expenses. Evaluating cost implications involves balancing initial packaging expenses against downstream sterilization and infection control costs to optimize budget and safety outcomes.
Choosing the Right Packaging for Medical Devices
Sterile packaging ensures medical devices remain free from microbial contamination, critical for preventing infections and maintaining patient safety during surgical and invasive procedures. Non-sterile packaging, suitable for devices that do not require aseptic conditions, offers cost-effective protection against physical damage and environmental factors. Selecting the right packaging depends on the device's intended use, regulatory requirements, and the necessity for maintaining sterility throughout the product's lifecycle.
Sterile packaging vs Non-sterile packaging Infographic
 productdif.com
productdif.com