The Unique Device Identification (UDI) system assigns a distinct identifier to medical devices to enhance traceability and patient safety, while the Global Unique Device Identification Database (GUDID) serves as the centralized repository where manufacturers submit detailed device information. UDI facilitates accurate device identification in clinical settings, and GUDID ensures regulatory authorities and healthcare providers have access to standardized data. Together, UDI and GUDID improve device tracking, recall efficiency, and post-market surveillance.
Table of Comparison
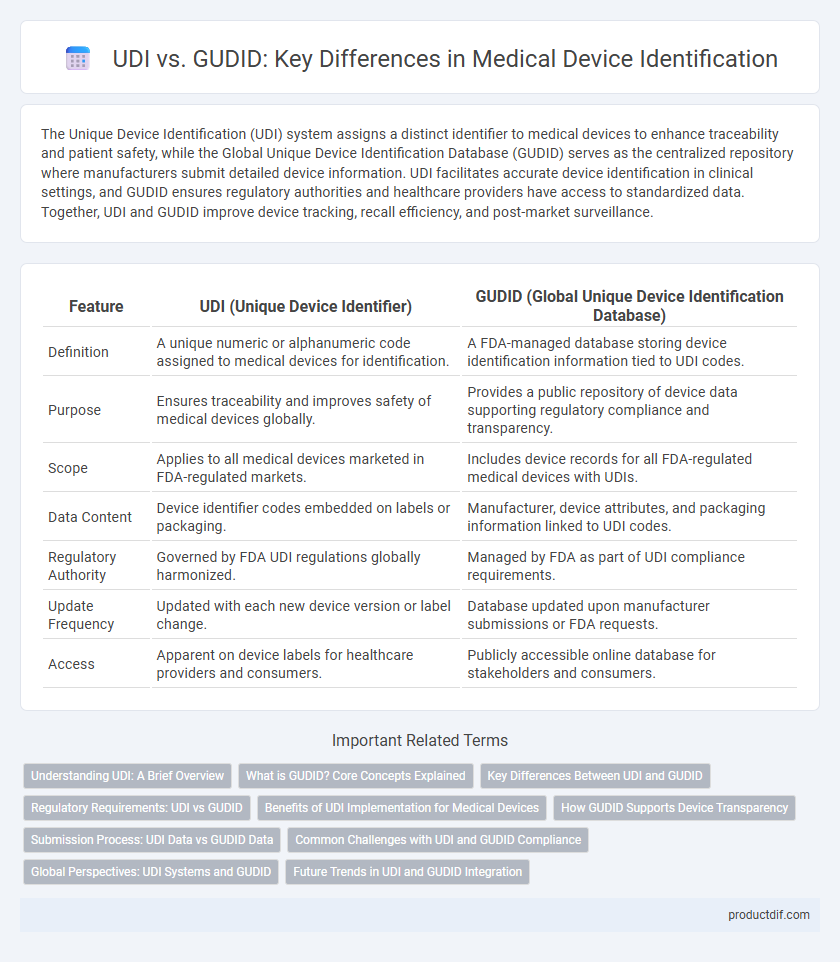
| Feature | UDI (Unique Device Identifier) | GUDID (Global Unique Device Identification Database) |
|---|---|---|
| Definition | A unique numeric or alphanumeric code assigned to medical devices for identification. | A FDA-managed database storing device identification information tied to UDI codes. |
| Purpose | Ensures traceability and improves safety of medical devices globally. | Provides a public repository of device data supporting regulatory compliance and transparency. |
| Scope | Applies to all medical devices marketed in FDA-regulated markets. | Includes device records for all FDA-regulated medical devices with UDIs. |
| Data Content | Device identifier codes embedded on labels or packaging. | Manufacturer, device attributes, and packaging information linked to UDI codes. |
| Regulatory Authority | Governed by FDA UDI regulations globally harmonized. | Managed by FDA as part of UDI compliance requirements. |
| Update Frequency | Updated with each new device version or label change. | Database updated upon manufacturer submissions or FDA requests. |
| Access | Apparent on device labels for healthcare providers and consumers. | Publicly accessible online database for stakeholders and consumers. |
Understanding UDI: A Brief Overview
Unique Device Identification (UDI) is a system designed to assign a distinct identifier to medical devices, enhancing traceability and patient safety. The Global Unique Device Identification Database (GUDID) serves as the FDA-maintained repository where manufacturers submit UDI data, ensuring comprehensive device information is accessible to stakeholders. Accurate UDI implementation and GUDID submission streamline regulatory compliance and improve post-market surveillance.
What is GUDID? Core Concepts Explained
GUDID, or the Global Unique Device Identification Database, is a comprehensive FDA-managed repository that stores key information on medical devices with UDI (Unique Device Identification) codes. It facilitates device identification, tracking, and post-market surveillance by providing standardized product data such as device identifiers, manufacturer details, and regulatory status. Accurate GUDID entries enhance patient safety, support recall management, and improve supply chain transparency in healthcare.
Key Differences Between UDI and GUDID
The Unique Device Identification (UDI) system assigns a unique code to medical devices for improving traceability, while the Global Unique Device Identification Database (GUDID) serves as the FDA-managed repository where all UDI data must be submitted. UDI consists of two parts: the Device Identifier (DI) and the Production Identifier (PI), whereas GUDID contains detailed device information such as manufacturer, brand, model, and device characteristics. UDI is the identification mechanism applied to the device, and GUDID is the centralized database ensuring regulated access and standardization of that identification data.
Regulatory Requirements: UDI vs GUDID
The Unique Device Identification (UDI) system mandates that medical devices carry a standardized identifier to enhance traceability and safety, while the Global Unique Device Identification Database (GUDID) serves as the FDA's centralized repository for submitting and accessing UDI data. Regulatory requirements for UDI focus on labeling standards and compliance deadlines, ensuring every medical device is publicly identifiable, whereas GUDID requirements emphasize the accurate and timely electronic submission of device information for transparency and post-market surveillance. Both UDI and GUDID regulations align under FDA mandates to improve device identification, reduce errors, and facilitate recalls and adverse event reporting efficiently.
Benefits of UDI Implementation for Medical Devices
UDI implementation for medical devices enhances patient safety by enabling precise device identification and traceability throughout the supply chain. Integration with the Global Unique Device Identification Database (GUDID) facilitates regulatory compliance, streamlines recalls, and improves adverse event reporting. This system also supports efficient inventory management and data analytics, aiding healthcare providers in clinical decision-making and reducing medical errors.
How GUDID Supports Device Transparency
The Global Unique Device Identification Database (GUDID) enhances medical device transparency by serving as a publicly accessible repository for Unique Device Identifiers (UDI), enabling healthcare providers and regulators to quickly verify device information. GUDID's comprehensive data supports improved tracking, safety monitoring, and regulatory compliance, facilitating faster identification of recalls and adverse events. This centralized transparency ensures accurate device traceability and promotes patient safety across healthcare systems.
Submission Process: UDI Data vs GUDID Data
The UDI submission process involves collecting comprehensive device identification data including device identifier (DI) and production identifier (PI) elements, which manufacturers must accurately compile. In contrast, GUDID submission specifically refers to the electronic transmission of the UDI data into the FDA's Global Unique Device Identification Database, ensuring regulatory compliance and public access. Effective UDI data management directly impacts the completeness and accuracy of GUDID entries, streamlining device traceability and post-market surveillance.
Common Challenges with UDI and GUDID Compliance
Healthcare providers and manufacturers face common challenges with UDI (Unique Device Identification) and GUDID (Global Unique Device Identification Database) compliance, such as accurately capturing and maintaining device identification data. Ensuring consistent data formatting and timely submission to the FDA's GUDID remains complex due to evolving regulatory requirements and diverse device configurations. Integration of UDI data into electronic health records and supply chain systems often encounters interoperability issues, impacting traceability and patient safety efforts.
Global Perspectives: UDI Systems and GUDID
UDI (Unique Device Identification) systems are implemented globally to enhance medical device traceability and patient safety, with significant variations across regions such as the US, EU, and Asia. The GUDID (Global Unique Device Identification Database) specifically serves as the centralized FDA repository for UDI data in the United States, enabling efficient access to device information by healthcare providers and regulators. International efforts toward harmonizing UDI systems and databases like GUDID aim to streamline regulatory compliance and facilitate global medical device surveillance.
Future Trends in UDI and GUDID Integration
Future trends in UDI and GUDID integration emphasize enhanced real-time data sharing between manufacturers, healthcare providers, and regulatory bodies to improve device traceability and patient safety. Advanced AI algorithms and blockchain technology are expected to drive automation in UDI data entry and verification within the GUDID system, reducing errors and increasing compliance. Increased global harmonization efforts aim to align UDI standards with GUDID databases, facilitating international market access and unified regulatory reporting.
UDI vs GUDID Infographic
 productdif.com
productdif.com