Class II medical devices are considered moderate risk and typically require special controls such as performance standards and postmarket surveillance, while Class III devices are high-risk and demand premarket approval with rigorous clinical data to ensure safety and efficacy. The regulatory pathway for Class III devices involves extensive trials and documentation compared to the often streamlined 510(k) clearance process used for Class II devices. Understanding the classification impacts design controls, regulatory requirements, and market entry strategies for medical device manufacturers.
Table of Comparison
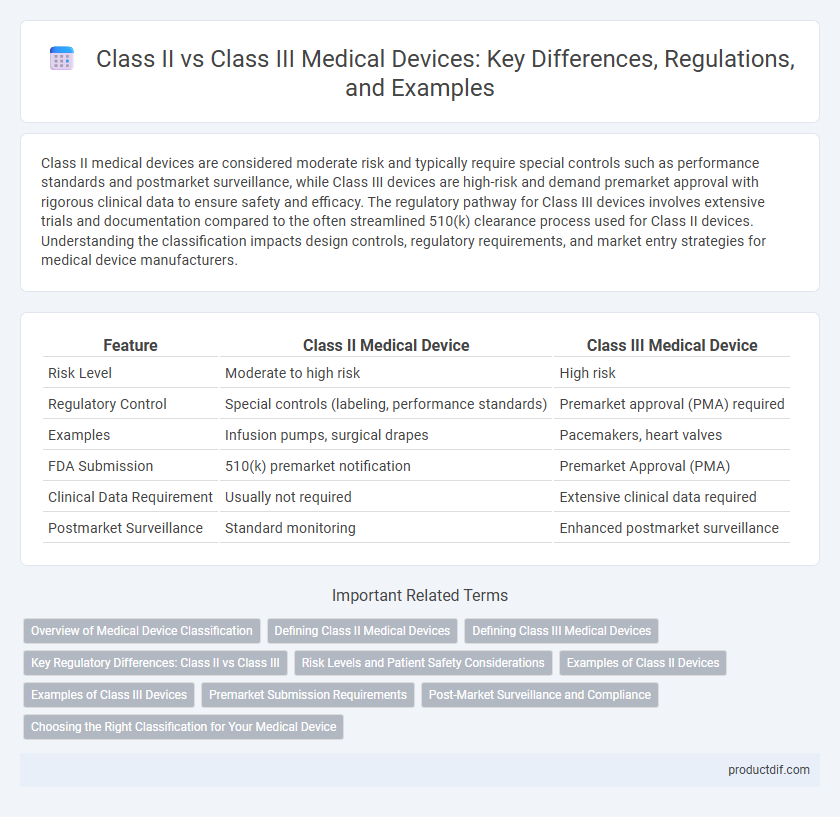
| Feature | Class II Medical Device | Class III Medical Device |
|---|---|---|
| Risk Level | Moderate to high risk | High risk |
| Regulatory Control | Special controls (labeling, performance standards) | Premarket approval (PMA) required |
| Examples | Infusion pumps, surgical drapes | Pacemakers, heart valves |
| FDA Submission | 510(k) premarket notification | Premarket Approval (PMA) |
| Clinical Data Requirement | Usually not required | Extensive clinical data required |
| Postmarket Surveillance | Standard monitoring | Enhanced postmarket surveillance |
Overview of Medical Device Classification
Class II medical devices pose moderate to high risk and require regulatory controls beyond general controls to ensure safety and effectiveness, including special labeling and performance standards. Class III devices are high-risk products, often life-sustaining or implantable, needing the most stringent premarket approval due to potential impact on patient health. Understanding the FDA classification system helps manufacturers comply with appropriate regulatory pathways based on device risk levels.
Defining Class II Medical Devices
Class II medical devices are defined by the FDA as products that require greater regulatory controls than Class I devices to ensure safety and effectiveness, typically involving moderate risk to patients. Examples include powered wheelchairs, infusion pumps, and surgical drapes, often subject to special labeling, performance standards, and postmarket surveillance. These devices usually require premarket notification [510(k)] clearance, distinguishing them from Class III devices that demand premarket approval (PMA) due to higher risk levels.
Defining Class III Medical Devices
Class III medical devices are defined by their critical role in sustaining or supporting life, posing a potential risk of illness or injury if they malfunction. These devices typically require premarket approval (PMA) due to their complexity and the high level of regulatory control necessary to ensure safety and effectiveness. Examples of Class III devices include implantable pacemakers, heart valves, and neurostimulators, which undergo rigorous clinical testing and FDA evaluation before market clearance.
Key Regulatory Differences: Class II vs Class III
Class II medical devices require special controls such as performance standards and postmarket surveillance, whereas Class III devices demand Premarket Approval (PMA) due to higher risk levels. Class III devices typically involve life-sustaining or life-supporting functions and undergo rigorous clinical trials to demonstrate safety and effectiveness. The regulatory pathway for Class III products is more stringent, involving extensive FDA scrutiny compared to the 510(k) premarket notification process for most Class II devices.
Risk Levels and Patient Safety Considerations
Class II medical devices present moderate to high risk and typically require special controls to ensure safety and effectiveness, including performance standards and postmarket surveillance. Class III devices involve the highest risk, supporting or sustaining human life, and mandate premarket approval with extensive clinical data to demonstrate safety and efficacy. Patient safety considerations for Class III devices are stringent due to their critical functions, necessitating rigorous regulatory scrutiny to minimize potential harm.
Examples of Class II Devices
Class II medical devices include powered wheelchairs, infusion pumps, diagnostic ultrasound systems, and surgical drapes, representing a wide range of moderate-risk products requiring greater regulatory control than Class I devices. These devices typically need premarket notification through the 510(k) process to demonstrate substantial equivalence to a legally marketed device. The regulatory focus emphasizes performance standards, postmarket surveillance, and special labeling to ensure safety and effectiveness in clinical use.
Examples of Class III Devices
Class III medical devices include implantable pacemakers, heart valves, and breast implants, which require rigorous premarket approval due to their high risk to patient health. These devices support or sustain human life and undergo extensive clinical testing to ensure safety and effectiveness. Unlike Class II devices, Class III devices are subject to the most stringent regulatory controls by authorities such as the FDA.
Premarket Submission Requirements
Class II medical devices typically require a 510(k) Premarket Notification demonstrating substantial equivalence to a legally marketed device, while Class III devices mandate a more rigorous Premarket Approval (PMA) process involving comprehensive clinical data to ensure safety and effectiveness. The PMA submission for Class III devices demands detailed scientific evidence, including extensive preclinical and clinical trial results, which often leads to longer review timelines compared to Class II 510(k) submissions. Compliance with these premarket submission requirements is critical for market clearance and regulatory approval under the U.S. Food and Drug Administration (FDA) framework.
Post-Market Surveillance and Compliance
Class II medical devices require moderate post-market surveillance focusing on routine compliance checks and adverse event reporting to ensure ongoing safety and effectiveness. Class III devices demand rigorous post-market surveillance, including detailed clinical follow-ups and enhanced reporting obligations due to their higher risk profile. Compliance for Class III devices involves strict adherence to regulatory standards with frequent audits and safety evaluations mandated by agencies like the FDA or EMA.
Choosing the Right Classification for Your Medical Device
Choosing the correct classification for your medical device ensures compliance with FDA regulations and affects the approval process duration; Class II devices typically require 510(k) premarket notification demonstrating substantial equivalence, while Class III devices undergo the more stringent Premarket Approval (PMA) process due to higher risk levels. Device risk, intended use, and technological characteristics are critical factors in determining whether a device fits Class II or Class III, guiding manufacturers toward the appropriate regulatory pathway. Accurate classification not only impacts market entry timelines but also influences post-market surveillance requirements and quality system controls essential for patient safety and product efficacy.
Class II vs Class III Infographic
 productdif.com
productdif.com