Custom medical devices for pets offer tailored solutions that address specific anatomical and health needs, enhancing treatment efficacy and comfort. Off-the-shelf devices provide immediate availability and lower cost but may lack the precise fit and functionality required for optimal outcomes. Choosing between custom and standard devices depends on the pet's condition, urgency of care, and budget constraints.
Table of Comparison
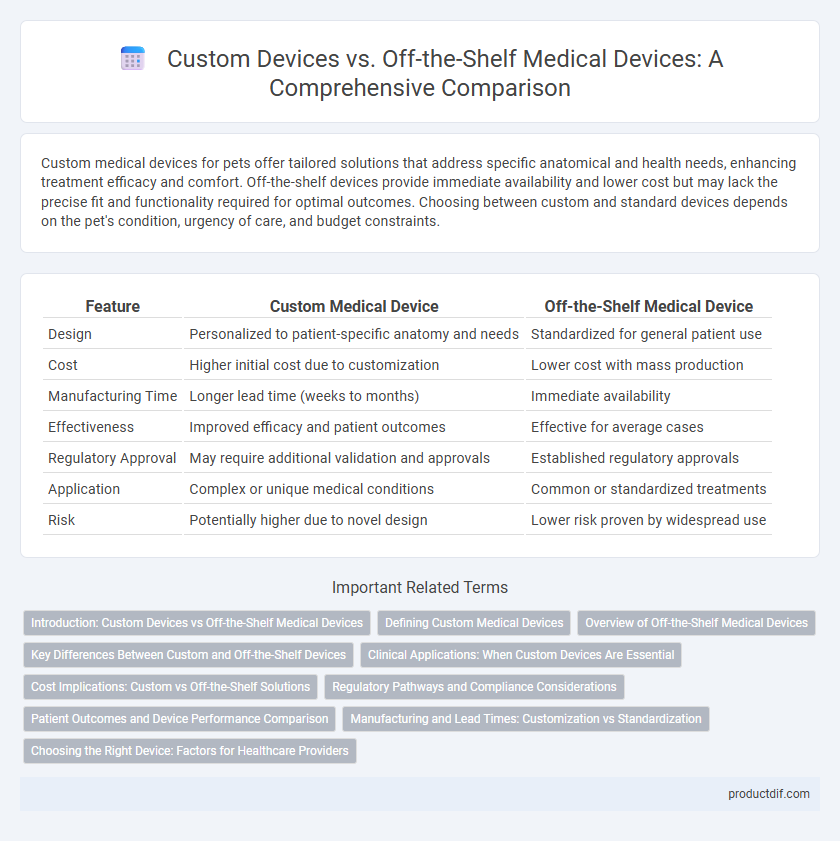
| Feature | Custom Medical Device | Off-the-Shelf Medical Device |
|---|---|---|
| Design | Personalized to patient-specific anatomy and needs | Standardized for general patient use |
| Cost | Higher initial cost due to customization | Lower cost with mass production |
| Manufacturing Time | Longer lead time (weeks to months) | Immediate availability |
| Effectiveness | Improved efficacy and patient outcomes | Effective for average cases |
| Regulatory Approval | May require additional validation and approvals | Established regulatory approvals |
| Application | Complex or unique medical conditions | Common or standardized treatments |
| Risk | Potentially higher due to novel design | Lower risk proven by widespread use |
Introduction: Custom Devices vs Off-the-Shelf Medical Devices
Custom medical devices offer tailored solutions designed to meet individual patient anatomy and clinical needs, enhancing treatment efficacy and patient comfort. Off-the-shelf medical devices provide standardized, mass-produced options with immediate availability and lower initial costs, suitable for common conditions and broad patient populations. Choosing between custom and off-the-shelf devices depends on factors such as complexity of medical condition, required precision, and budget constraints.
Defining Custom Medical Devices
Custom medical devices are specifically designed and manufactured to meet the unique anatomical and functional requirements of individual patients, ensuring precise fit and optimal therapeutic outcomes. Unlike off-the-shelf devices, which are mass-produced in standard sizes and configurations, custom devices undergo a personalized development process involving detailed patient data, advanced imaging, and tailored materials. Regulatory frameworks, such as the FDA's guidelines, distinctly categorize custom devices due to their bespoke nature, emphasizing the importance of compliance in design, manufacturing, and clinical application.
Overview of Off-the-Shelf Medical Devices
Off-the-shelf medical devices are pre-manufactured products designed for general use across a wide patient population, offering immediate availability and standardized features. These devices include items such as pacemakers, orthopedic implants, and diagnostic equipment that meet regulatory standards like FDA approval. Their cost-effectiveness and consistent quality make them preferable for healthcare providers seeking reliable solutions without the delay of customization.
Key Differences Between Custom and Off-the-Shelf Devices
Custom medical devices offer tailored solutions designed specifically for individual patients' anatomical and physiological needs, ensuring higher precision and better clinical outcomes. Off-the-shelf devices are mass-produced, standardized products available for immediate use, offering cost efficiency and widespread availability but limited customization. Key differences include personalization, lead time, cost, and regulatory approval pathways, with custom devices often requiring longer development times and specialized regulatory assessments.
Clinical Applications: When Custom Devices Are Essential
Custom medical devices play a crucial role in clinical applications where patient-specific anatomy and unique medical conditions demand tailored solutions, ensuring optimal fit and functionality. Off-the-shelf devices may fall short in cases such as complex orthopedic implants, personalized prosthetics, and specialized surgical tools requiring precise customization. Leveraging advanced manufacturing technologies like 3D printing, custom devices enhance surgical outcomes and patient recovery by addressing individual clinical needs effectively.
Cost Implications: Custom vs Off-the-Shelf Solutions
Custom medical devices typically involve higher upfront costs due to personalized design, specialized materials, and tailored manufacturing processes. Off-the-shelf solutions benefit from mass production and economies of scale, resulting in lower initial prices and faster availability. Long-term cost implications also vary, with custom devices potentially reducing patient complications and readmissions, impacting overall healthcare expenses.
Regulatory Pathways and Compliance Considerations
Custom medical devices require a tailored regulatory pathway, typically involving the FDA's Custom Device Exemption (CDE), which provides some flexibility but demands strict documentation and justification for individual patient use. Off-the-shelf devices must comply with established regulatory standards such as FDA clearance through 510(k) premarket notification or Premarket Approval (PMA), ensuring broader market access but less design flexibility. Manufacturers must carefully navigate compliance considerations including quality system regulations, labeling, and post-market surveillance specific to each device category to meet safety and efficacy requirements.
Patient Outcomes and Device Performance Comparison
Custom medical devices tailored to an individual's anatomy often result in improved patient outcomes by enhancing device fit, comfort, and functionality. Off-the-shelf devices, while more readily available and cost-effective, may sometimes lead to suboptimal performance due to generic sizing and design limitations. Studies indicate that custom devices can reduce complication rates and improve long-term device efficacy, directly impacting recovery times and patient satisfaction.
Manufacturing and Lead Times: Customization vs Standardization
Custom medical devices require tailored manufacturing processes involving precise design adjustments, which extend lead times due to prototyping and validation phases. Off-the-shelf devices benefit from standardized production lines, enabling faster availability and reduced costs through economies of scale. Balancing customization with lead time efficiency is crucial for meeting specific patient needs without compromising timely delivery.
Choosing the Right Device: Factors for Healthcare Providers
Healthcare providers must evaluate patient-specific needs, anatomical variations, and clinical outcomes when choosing between custom devices and off-the-shelf medical devices. Custom devices offer tailored fit and enhanced functionality, especially for complex cases, while off-the-shelf options provide cost efficiency and faster availability. Decision-making also involves considering regulatory compliance, manufacturing timelines, and long-term patient benefits.
Custom device vs Off-the-shelf Infographic
 productdif.com
productdif.com