Biocompatibility in medical devices ensures that materials interact safely with biological tissues without causing adverse reactions, such as inflammation or toxicity. Non-biocompatible materials can trigger immune responses or tissue damage, leading to device failure or patient harm. Selecting biocompatible materials is critical for long-term implant success and patient safety.
Table of Comparison
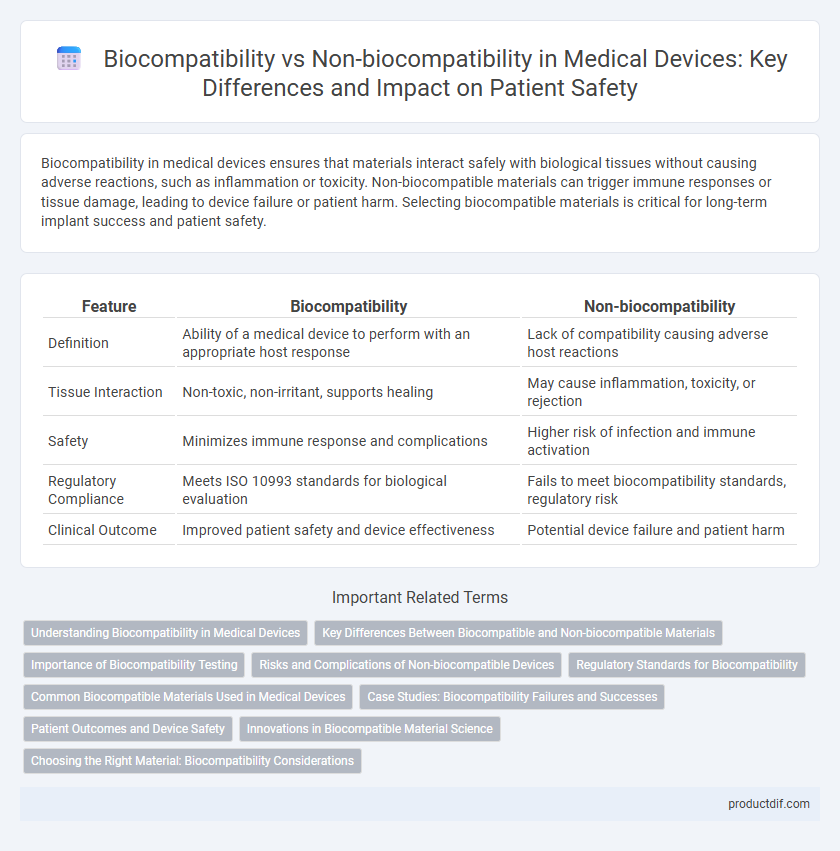
| Feature | Biocompatibility | Non-biocompatibility |
|---|---|---|
| Definition | Ability of a medical device to perform with an appropriate host response | Lack of compatibility causing adverse host reactions |
| Tissue Interaction | Non-toxic, non-irritant, supports healing | May cause inflammation, toxicity, or rejection |
| Safety | Minimizes immune response and complications | Higher risk of infection and immune activation |
| Regulatory Compliance | Meets ISO 10993 standards for biological evaluation | Fails to meet biocompatibility standards, regulatory risk |
| Clinical Outcome | Improved patient safety and device effectiveness | Potential device failure and patient harm |
Understanding Biocompatibility in Medical Devices
Biocompatibility in medical devices refers to the material's ability to interact with the human body without causing adverse reactions, ensuring safety and effectiveness during implantation or contact with tissues. Non-biocompatible materials can trigger immune responses, inflammation, or toxicity, compromising patient health and device performance. Rigorous testing standards such as ISO 10993 guide the evaluation of biocompatibility to mitigate risks associated with medical device material selection.
Key Differences Between Biocompatible and Non-biocompatible Materials
Biocompatible materials are designed to interact safely with biological tissues without causing adverse immune responses, making them essential for implantable medical devices, prosthetics, and contact lenses. Non-biocompatible materials, in contrast, can trigger inflammation, toxicity, or rejection when in contact with body tissues, limiting their use to external applications or requiring additional coatings to reduce incompatibility. Key differences include cellular response, toxicity levels, and long-term stability within the human body, which critically impact device safety and functionality.
Importance of Biocompatibility Testing
Biocompatibility testing is essential for medical devices to ensure they do not cause adverse reactions when in contact with human tissues or bodily fluids. This testing evaluates the compatibility of materials, preventing complications such as inflammation, toxicity, or immune responses. Regulatory agencies require rigorous biocompatibility assessments to guarantee patient safety and device efficacy.
Risks and Complications of Non-biocompatible Devices
Non-biocompatible medical devices can trigger severe immune responses, including inflammation, allergic reactions, and chronic tissue damage, significantly increasing patient risk. These devices often cause complications such as implant rejection, infection, and fibrosis, leading to device failure and potential need for surgical removal. Prolonged exposure to non-biocompatible materials may result in systemic toxicity or carcinogenic effects, compromising overall patient safety and treatment outcomes.
Regulatory Standards for Biocompatibility
Regulatory standards for biocompatibility, such as ISO 10993 and FDA guidance, establish rigorous testing criteria to ensure medical devices do not cause adverse biological reactions when in contact with patients. These standards require evaluation of cytotoxicity, sensitization, and systemic toxicity to classify materials as biocompatible or non-biocompatible. Compliance with these protocols is critical for device approval, patient safety, and market access in global healthcare systems.
Common Biocompatible Materials Used in Medical Devices
Common biocompatible materials used in medical devices include titanium, stainless steel, silicone, polyethylene, and certain ceramics due to their ability to minimize immune response and promote tissue integration. These materials demonstrate excellent corrosion resistance, mechanical strength, and bio-inert properties essential for implants, prosthetics, and surgical instruments. In contrast, non-biocompatible materials often induce inflammation, cytotoxicity, or rejection, limiting their application in direct patient contact.
Case Studies: Biocompatibility Failures and Successes
Numerous case studies highlight the critical impact of biocompatibility on medical device performance, where failures often lead to severe inflammatory responses, implant rejection, or systemic toxicity. Successful biocompatible devices, such as certain cardiovascular stents and orthopedic implants, demonstrate enhanced patient outcomes by minimizing adverse immune reactions through advanced material selection and surface engineering. Analysis of biocompatibility failures emphasizes the importance of rigorous preclinical testing and long-term monitoring to prevent complications and improve device safety profiles.
Patient Outcomes and Device Safety
Biocompatibility in medical devices ensures materials do not provoke adverse immune responses, significantly improving patient outcomes by reducing inflammation, infection, and device rejection. Non-biocompatible devices increase risks of complications, leading to prolonged recovery times and potentially life-threatening conditions. Prioritizing biocompatible materials enhances device safety and long-term functionality, critical for successful clinical applications.
Innovations in Biocompatible Material Science
Innovations in biocompatible material science have revolutionized medical device development by enhancing tissue integration and reducing immune response risks. Advanced polymers and bioactive ceramics demonstrate superior compatibility, promoting faster healing and minimizing inflammation compared to non-biocompatible materials. These breakthroughs support safer, longer-lasting implants and enable the creation of next-generation devices with improved patient outcomes.
Choosing the Right Material: Biocompatibility Considerations
Selecting materials for medical devices requires prioritizing biocompatibility to ensure minimal adverse reactions when in contact with bodily tissues. Biocompatible materials like titanium, medical-grade silicone, and certain polymers exhibit favorable integration and reduced inflammation risk, whereas non-biocompatible materials may cause toxicity, immune response, or device failure. Comprehensive evaluation of material properties, including cytotoxicity, sensitization, and chronic toxicity, guides the optimal choice for safe and effective medical device performance.
Biocompatibility vs Non-biocompatibility Infographic
 productdif.com
productdif.com