Biocompatible materials interact positively with surrounding tissues, promoting healing and minimizing immune response, making them ideal for implantable medical devices. In contrast, bioinert materials are designed to remain stable without eliciting significant biological reactions, ensuring durability and mechanical integrity within the body. Selecting between biocompatible and bioinert materials depends on the device's intended function and required tissue integration.
Table of Comparison
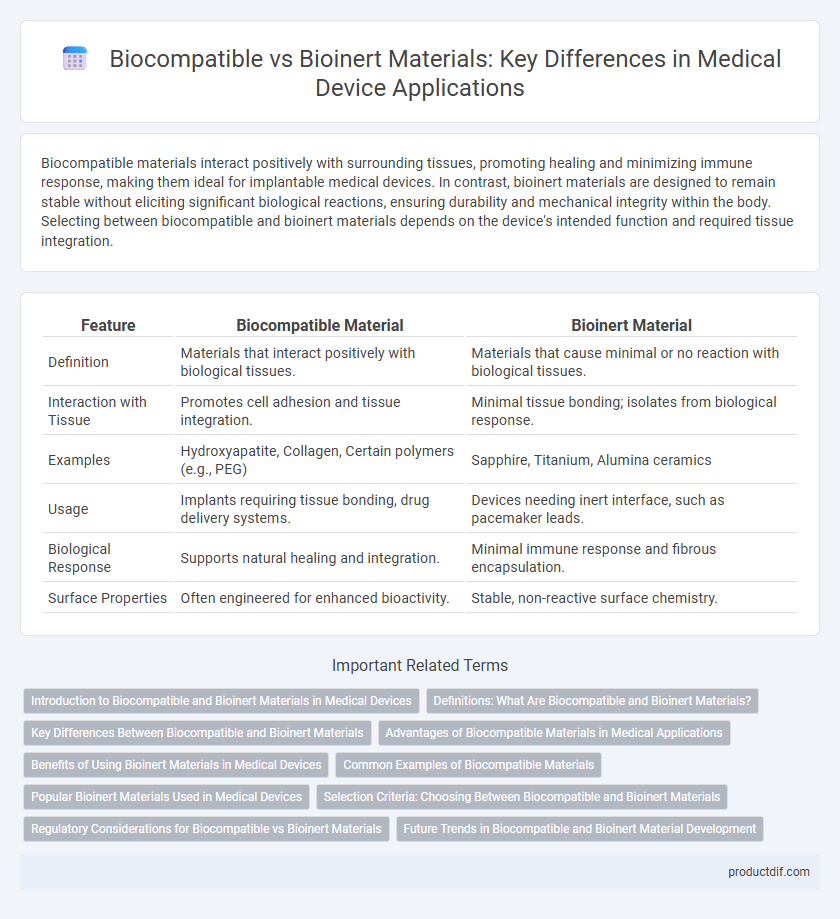
| Feature | Biocompatible Material | Bioinert Material |
|---|---|---|
| Definition | Materials that interact positively with biological tissues. | Materials that cause minimal or no reaction with biological tissues. |
| Interaction with Tissue | Promotes cell adhesion and tissue integration. | Minimal tissue bonding; isolates from biological response. |
| Examples | Hydroxyapatite, Collagen, Certain polymers (e.g., PEG) | Sapphire, Titanium, Alumina ceramics |
| Usage | Implants requiring tissue bonding, drug delivery systems. | Devices needing inert interface, such as pacemaker leads. |
| Biological Response | Supports natural healing and integration. | Minimal immune response and fibrous encapsulation. |
| Surface Properties | Often engineered for enhanced bioactivity. | Stable, non-reactive surface chemistry. |
Introduction to Biocompatible and Bioinert Materials in Medical Devices
Biocompatible materials in medical devices interact harmoniously with biological tissues, minimizing immune responses and ensuring device functionality. Bioinert materials, typically composed of ceramics or metals, maintain stability within the body by avoiding chemical or biological reactions, thereby reducing the risk of inflammation or rejection. Selecting between biocompatible and bioinert materials depends on the device's application, intended duration of implantation, and the body's response to foreign objects.
Definitions: What Are Biocompatible and Bioinert Materials?
Biocompatible materials are substances designed to interact with biological systems without causing adverse effects, supporting tissue integration and promoting healing. Bioinert materials, a subset of biocompatible materials, exhibit minimal or no reaction with surrounding tissues, maintaining stability without eliciting a biological response. Understanding the distinction between these materials is critical for selecting appropriate components in medical devices to optimize patient safety and device performance.
Key Differences Between Biocompatible and Bioinert Materials
Biocompatible materials interact positively with biological systems, promoting tissue integration and minimizing immune response, while bioinert materials are designed to be chemically stable and minimize interaction with surrounding tissues, reducing the risk of inflammation. Biocompatible materials often degrade or adapt to the biological environment, whereas bioinert materials maintain their physical and chemical properties without altering the tissue environment. The choice between these materials depends on the medical device application, with biocompatible materials preferred for implants requiring integration, and bioinert materials favored for long-term stability without biological interference.
Advantages of Biocompatible Materials in Medical Applications
Biocompatible materials offer superior integration with human tissues, reducing immune responses and promoting faster healing in medical implants. Their ability to interact positively with biological systems minimizes inflammation and enhances long-term device functionality. This makes biocompatible materials preferable for applications such as prosthetics, cardiovascular stents, and tissue engineering scaffolds.
Benefits of Using Bioinert Materials in Medical Devices
Bioinert materials exhibit exceptional stability and minimal biological reactivity, reducing the risk of adverse immune responses when used in medical devices. Their ability to maintain structural integrity and avoid corrosion ensures long-term durability in implants and prosthetics. These properties make bioinert materials ideal for applications requiring consistent performance and compatibility within the human body.
Common Examples of Biocompatible Materials
Common examples of biocompatible materials include titanium, medical-grade stainless steel, and certain polymers such as polyethylene and silicone. These materials interact favorably with biological tissues, minimizing immune response and promoting integration within the body. Biocompatible materials are essential in implants, prosthetics, and surgical instruments to ensure safety and functionality in medical applications.
Popular Bioinert Materials Used in Medical Devices
Popular bioinert materials used in medical devices include titanium, alumina, and certain grades of stainless steel, prized for their excellent corrosion resistance and stable interaction with biological tissues. These materials minimize immune response and inflammation, making them ideal for implants such as joint replacements, dental implants, and cardiovascular devices. Their mechanical strength combined with bioinert properties ensures long-term durability and patient safety in various medical applications.
Selection Criteria: Choosing Between Biocompatible and Bioinert Materials
Selection criteria for choosing between biocompatible and bioinert materials in medical devices depend on the specific application and tissue interaction required. Biocompatible materials are preferred when integration with surrounding tissues or cellular response modulation is essential, while bioinert materials are ideal for implant surfaces that must minimize immune reaction and avoid fibrous encapsulation. Factors such as mechanical properties, degradation rates, and the potential for inflammation guide the optimal material choice to ensure device safety and effectiveness.
Regulatory Considerations for Biocompatible vs Bioinert Materials
Regulatory considerations for biocompatible materials emphasize rigorous biocompatibility testing, including cytotoxicity, sensitization, and systemic toxicity assessments to ensure patient safety and compliance with standards like ISO 10993. In contrast, bioinert materials, characterized by minimal tissue interaction, require validation of chemical stability and inertness under physiological conditions, often streamlining regulatory approval but still necessitating conformity with specific FDA or EMA guidelines. Both material types demand thorough documentation of manufacturing processes, traceability, and post-market surveillance to address potential adverse reactions and maintain regulatory compliance.
Future Trends in Biocompatible and Bioinert Material Development
Future trends in biocompatible and bioinert material development emphasize advanced surface modification techniques and nanostructuring to enhance cellular integration and reduce immune responses. Innovations in smart biomaterials with responsive properties enable real-time interaction with biological environments, improving device functionality and patient outcomes. Research is also directed toward biodegradable bioinert composites that combine mechanical strength with controlled degradation for temporary medical implants.
Biocompatible Material vs Bioinert Material Infographic
 productdif.com
productdif.com