Design controls ensure medical devices meet user needs and regulatory requirements through structured design, verification, and validation activities. Process controls monitor and maintain the manufacturing process to guarantee consistent product quality and compliance during production. Both controls are essential for delivering safe, effective medical devices and achieving regulatory approval.
Table of Comparison
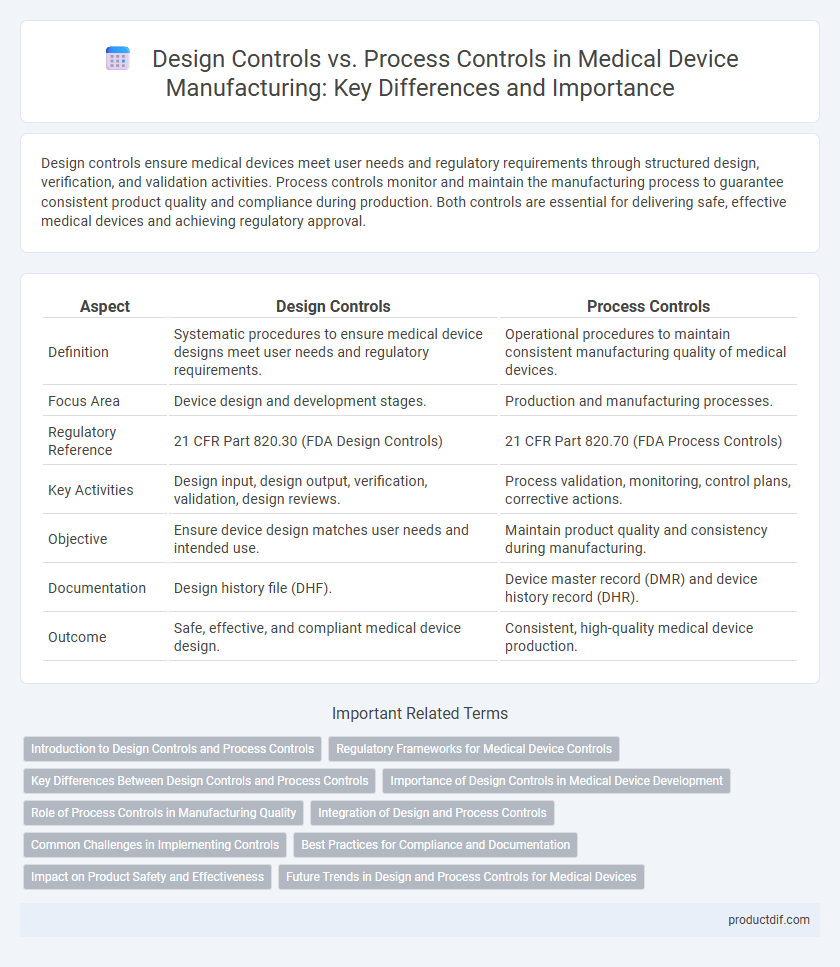
| Aspect | Design Controls | Process Controls |
|---|---|---|
| Definition | Systematic procedures to ensure medical device designs meet user needs and regulatory requirements. | Operational procedures to maintain consistent manufacturing quality of medical devices. |
| Focus Area | Device design and development stages. | Production and manufacturing processes. |
| Regulatory Reference | 21 CFR Part 820.30 (FDA Design Controls) | 21 CFR Part 820.70 (FDA Process Controls) |
| Key Activities | Design input, design output, verification, validation, design reviews. | Process validation, monitoring, control plans, corrective actions. |
| Objective | Ensure device design matches user needs and intended use. | Maintain product quality and consistency during manufacturing. |
| Documentation | Design history file (DHF). | Device master record (DMR) and device history record (DHR). |
| Outcome | Safe, effective, and compliant medical device design. | Consistent, high-quality medical device production. |
Introduction to Design Controls and Process Controls
Design Controls establish a structured framework ensuring medical devices meet user needs and regulatory requirements through systematic planning, design input, output, verification, validation, and design reviews. Process Controls focus on monitoring and controlling manufacturing activities to maintain product quality and consistency, including equipment calibration, process validation, and standard operating procedures. Together, these controls ensure compliance with FDA 21 CFR Part 820 and ISO 13485 standards for medical device safety and effectiveness.
Regulatory Frameworks for Medical Device Controls
Design controls in medical devices focus on systematic procedures for product development, ensuring compliance with FDA 21 CFR 820.30 and ISO 13485 standards. Process controls emphasize monitoring and managing manufacturing operations to maintain consistent quality and meet regulatory requirements such as FDA QSR and MDSAP guidelines. Both controls are integral to regulatory frameworks, ensuring safety, efficacy, and traceability throughout the device lifecycle.
Key Differences Between Design Controls and Process Controls
Design controls concentrate on planning, documenting, and verifying medical device specifications to ensure product safety and effectiveness, while process controls focus on monitoring and managing manufacturing activities to maintain consistent quality. Design controls involve stages such as design inputs, outputs, verification, and validation, whereas process controls emphasize process validation, equipment calibration, and in-process inspections. Both controls are essential for regulatory compliance under FDA 21 CFR 820, but design controls address product development, and process controls target production stability.
Importance of Design Controls in Medical Device Development
Design controls establish a structured framework ensuring medical devices meet user needs, safety standards, and regulatory requirements before production begins. Implementing robust design controls minimizes risks, prevents costly errors, and facilitates traceability throughout the product lifecycle. This process is critical for achieving FDA compliance and maintaining product quality in complex medical device development.
Role of Process Controls in Manufacturing Quality
Process controls in medical device manufacturing ensure consistent product quality by regulating production parameters such as temperature, pressure, and machine calibration throughout the manufacturing process. These controls minimize variability and defects, leading to greater reliability and compliance with regulatory standards like FDA's 21 CFR Part 820. Unlike design controls that focus on product development and validation, process controls maintain ongoing operational quality, enabling real-time monitoring and corrective actions to uphold device safety and performance.
Integration of Design and Process Controls
Integration of Design Controls and Process Controls in medical device manufacturing ensures seamless transition from product development to production, enhancing regulatory compliance and product quality. Design Controls focus on requirements, design inputs, and verification, while Process Controls emphasize manufacturing efficiency, validation, and monitoring. Combining these controls facilitates traceability, risk management, and continuous improvement across the device lifecycle.
Common Challenges in Implementing Controls
Design Controls and Process Controls in medical device manufacturing often face challenges such as inadequate documentation, unclear responsibility assignments, and lack of standardized procedures. Inconsistent verification and validation processes can result in regulatory non-compliance and product quality issues. Ensuring proper integration between design and process controls requires continuous training and robust communication across cross-functional teams.
Best Practices for Compliance and Documentation
Design controls in medical device development emphasize structured documentation of user needs, design inputs, and verification activities to ensure regulatory compliance, while process controls focus on monitoring and controlling manufacturing processes to maintain product quality. Best practices include maintaining traceability matrices, conducting risk assessments aligned with ISO 13485 and FDA 21 CFR Part 820 standards, and implementing robust change management systems. Comprehensive documentation that integrates design and process controls supports audit readiness and facilitates continuous improvement in product safety and effectiveness.
Impact on Product Safety and Effectiveness
Design controls establish systematic procedures to ensure medical devices meet specified safety and effectiveness requirements throughout development, directly reducing risks related to device functionality and patient harm. Process controls oversee manufacturing consistency, ensuring that each device produced adheres to quality standards that maintain performance reliability and compliance with regulatory requirements. The integration of both design and process controls is critical in minimizing variability, preventing defects, and safeguarding patient outcomes.
Future Trends in Design and Process Controls for Medical Devices
Future trends in design and process controls for medical devices emphasize increased integration of artificial intelligence and machine learning to enhance product validation and risk management. Regulatory frameworks are evolving to demand greater real-time monitoring and traceability throughout the product lifecycle, ensuring compliance and patient safety. Advanced digital twins and predictive analytics are set to revolutionize both design verification and manufacturing process optimization.
Design Controls vs Process Controls Infographic
 productdif.com
productdif.com