Wet lab testing in medical device development involves hands-on experiments with biological samples and chemical reagents to evaluate device functionality under realistic physiological conditions. Dry lab testing relies on computer simulations and data analysis to predict device performance and optimize design without physical prototypes. Combining both approaches enhances accuracy and reduces development time by validating computational models with experimental data.
Table of Comparison
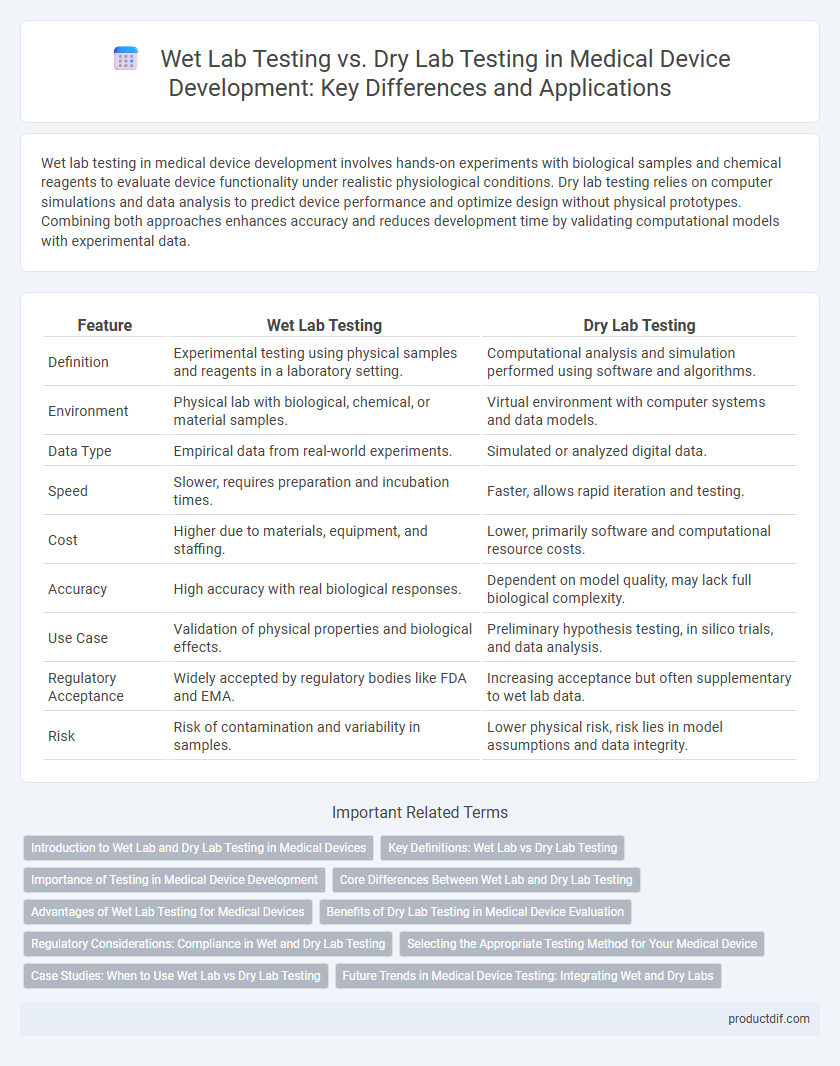
| Feature | Wet Lab Testing | Dry Lab Testing |
|---|---|---|
| Definition | Experimental testing using physical samples and reagents in a laboratory setting. | Computational analysis and simulation performed using software and algorithms. |
| Environment | Physical lab with biological, chemical, or material samples. | Virtual environment with computer systems and data models. |
| Data Type | Empirical data from real-world experiments. | Simulated or analyzed digital data. |
| Speed | Slower, requires preparation and incubation times. | Faster, allows rapid iteration and testing. |
| Cost | Higher due to materials, equipment, and staffing. | Lower, primarily software and computational resource costs. |
| Accuracy | High accuracy with real biological responses. | Dependent on model quality, may lack full biological complexity. |
| Use Case | Validation of physical properties and biological effects. | Preliminary hypothesis testing, in silico trials, and data analysis. |
| Regulatory Acceptance | Widely accepted by regulatory bodies like FDA and EMA. | Increasing acceptance but often supplementary to wet lab data. |
| Risk | Risk of contamination and variability in samples. | Lower physical risk, risk lies in model assumptions and data integrity. |
Introduction to Wet Lab and Dry Lab Testing in Medical Devices
Wet lab testing in medical devices involves the use of biological materials, chemicals, or fluids to simulate real-world conditions and assess device performance, biocompatibility, and safety under physiological environments. Dry lab testing utilizes computer simulations, mathematical modeling, and digital analysis to predict device behavior, optimize design, and perform virtual trials without the need for physical specimens or fluids. Integrating both wet and dry lab testing enables comprehensive evaluation, regulatory compliance, and accelerated innovation in medical device development.
Key Definitions: Wet Lab vs Dry Lab Testing
Wet lab testing in medical device development involves experimental procedures using biological samples, chemicals, or reagents to analyze device performance under real-world physiological conditions. Dry lab testing relies on computational simulations, data modeling, and virtual environments to predict device behavior without physical experimentation. Both approaches are critical for validating safety, efficacy, and regulatory compliance, with wet labs offering empirical data and dry labs providing cost-effective, rapid prototyping insights.
Importance of Testing in Medical Device Development
Wet lab testing in medical device development involves experimental procedures using biological materials to evaluate device safety, functionality, and biocompatibility under real-world conditions. Dry lab testing utilizes computer simulations and computational models to predict device performance, reducing time and cost while enhancing design accuracy. Both testing methods are crucial for regulatory approval, ensuring reliability, efficacy, and patient safety before clinical application.
Core Differences Between Wet Lab and Dry Lab Testing
Wet lab testing in medical device development involves handling biological samples, reagents, and chemicals to conduct experiments under controlled physical conditions, ensuring realistic simulation of physiological environments. Dry lab testing primarily relies on computational models, simulations, and data analysis to predict device performance without direct interaction with biological materials, enabling quicker iterations and cost efficiency. The core differences lie in the tangible experimental approach of wet labs versus the virtual, software-driven focus of dry labs, impacting validation processes and development timelines.
Advantages of Wet Lab Testing for Medical Devices
Wet lab testing for medical devices offers highly accurate simulation of physiological conditions, enabling precise evaluation of device biocompatibility and functionality. This testing method allows for direct interaction with biological tissues and fluids, providing critical insights into real-world performance and safety. Enhanced data reliability from wet lab experiments supports regulatory approval and reduces clinical trial risks.
Benefits of Dry Lab Testing in Medical Device Evaluation
Dry lab testing in medical device evaluation offers increased efficiency by enabling rapid simulation and data analysis without the need for physical prototypes. It reduces costs significantly by minimizing material consumption and resource allocation for experimental setups. Dry lab methods also facilitate early identification of design flaws through advanced modeling, improving overall product safety and effectiveness.
Regulatory Considerations: Compliance in Wet and Dry Lab Testing
Regulatory considerations for wet lab testing in medical device development emphasize strict compliance with biosafety standards, specimen handling protocols, and contamination controls mandated by agencies like the FDA and ISO 13485. Dry lab testing prioritizes data integrity, software validation, and cybersecurity measures to meet regulatory requirements for computer-simulated experiments and electronic data management systems. Both testing environments require comprehensive documentation and adherence to Good Laboratory Practice (GLP) guidelines to ensure traceability and reproducibility in regulatory submissions.
Selecting the Appropriate Testing Method for Your Medical Device
Selecting the appropriate testing method for your medical device depends on the device's intended use, complexity, and validation requirements. Wet lab testing involves real biological samples or fluids to simulate clinical conditions, providing critical data on biocompatibility and device performance. Dry lab testing utilizes computer simulations and data analysis, offering a cost-effective and rapid approach for design optimization and preliminary verification.
Case Studies: When to Use Wet Lab vs Dry Lab Testing
Case studies in medical device development reveal that wet lab testing is essential for evaluating biocompatibility and fluid dynamics in realistic physiological conditions, providing hands-on insight into device-material interactions. Dry lab testing excels in early-stage simulations and computational modeling, offering cost-effective optimization of device design and performance without physical prototypes. Selecting wet versus dry lab testing depends on project phase, goals, and whether biological factors or algorithmic accuracy need validation.
Future Trends in Medical Device Testing: Integrating Wet and Dry Labs
Future trends in medical device testing emphasize the integration of wet lab testing and dry lab testing to enhance accuracy and efficiency. Combining wet labs' experimental validation with dry labs' computational modeling and simulation enables more comprehensive evaluation of device safety and performance. This hybrid approach accelerates development cycles and supports regulatory compliance through robust, data-driven insights.
Wet lab testing vs Dry lab testing Infographic
 productdif.com
productdif.com