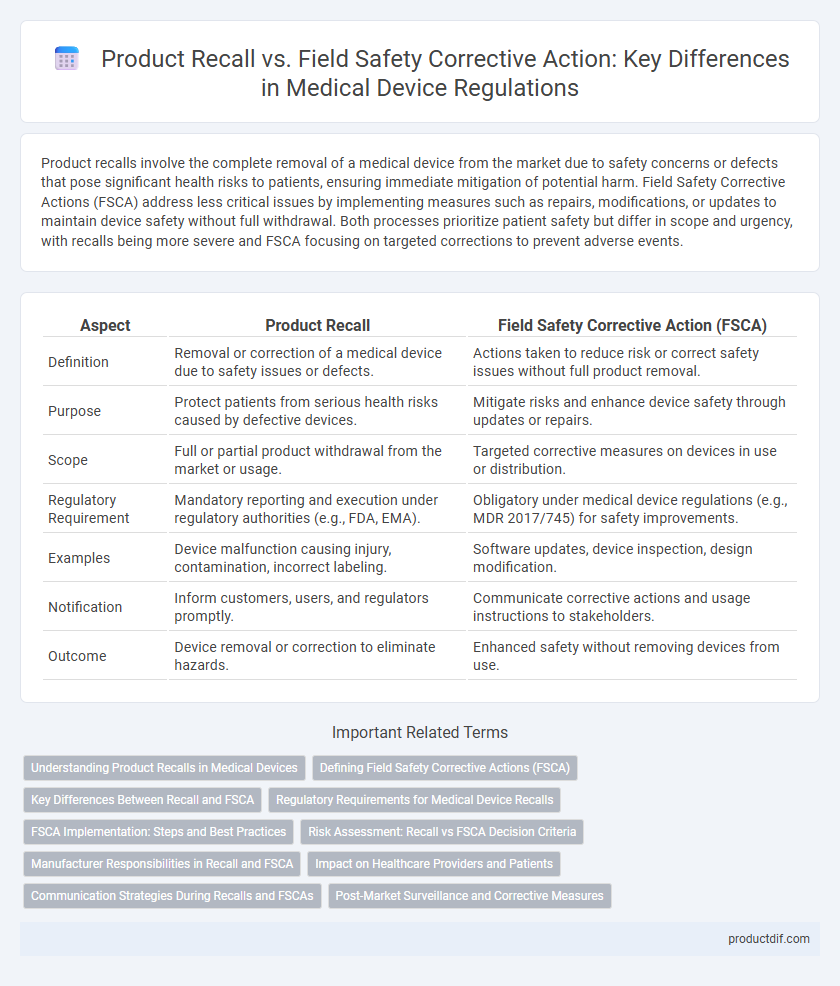
Product recalls involve the complete removal of a medical device from the market due to safety concerns or defects that pose significant health risks to patients, ensuring immediate mitigation of potential harm. Field Safety Corrective Actions (FSCA) address less critical issues by implementing measures such as repairs, modifications, or updates to maintain device safety without full withdrawal. Both processes prioritize patient safety but differ in scope and urgency, with recalls being more severe and FSCA focusing on targeted corrections to prevent adverse events.
Table of Comparison
| Aspect | Product Recall | Field Safety Corrective Action (FSCA) |
|---|---|---|
| Definition | Removal or correction of a medical device due to safety issues or defects. | Actions taken to reduce risk or correct safety issues without full product removal. |
| Purpose | Protect patients from serious health risks caused by defective devices. | Mitigate risks and enhance device safety through updates or repairs. |
| Scope | Full or partial product withdrawal from the market or usage. | Targeted corrective measures on devices in use or distribution. |
| Regulatory Requirement | Mandatory reporting and execution under regulatory authorities (e.g., FDA, EMA). | Obligatory under medical device regulations (e.g., MDR 2017/745) for safety improvements. |
| Examples | Device malfunction causing injury, contamination, incorrect labeling. | Software updates, device inspection, design modification. |
| Notification | Inform customers, users, and regulators promptly. | Communicate corrective actions and usage instructions to stakeholders. |
| Outcome | Device removal or correction to eliminate hazards. | Enhanced safety without removing devices from use. |
Understanding Product Recalls in Medical Devices
Product recalls in medical devices involve removing or correcting products that pose a potential health risk, driven by regulatory authorities like the FDA or EMA to ensure patient safety. Field Safety Corrective Actions (FSCAs) specifically address issues detected post-market without necessarily removing the product, focusing on mitigation or correction while maintaining device availability. Understanding the distinctions and regulatory requirements between recalls and FSCAs is critical for manufacturers to comply with safety standards and limit liability.
Defining Field Safety Corrective Actions (FSCA)
Field Safety Corrective Actions (FSCA) refer to measures taken by medical device manufacturers to reduce the risk of harm associated with a device already on the market, often involving updates, repairs, or instructions for safe use. FSCAs are distinct from product recalls as they are typically less severe interventions aimed at mitigating safety risks without removing the device from the market entirely. Regulatory bodies like the FDA and the European Medicines Agency require manufacturers to implement FSCAs promptly to ensure ongoing patient safety and device compliance.
Key Differences Between Recall and FSCA
A Product Recall involves the removal or correction of a medical device that poses serious health risks, often initiated by regulatory authorities due to non-compliance or safety concerns. Field Safety Corrective Action (FSCA) is a targeted measure taken by manufacturers to reduce the risk associated with a device already distributed, focusing on user notification and specific risk mitigation without necessarily removing the product. Key differences include the scope and severity, where recalls address critical hazards requiring device withdrawal, while FSCAs manage lower-risk issues through corrective notifications and updates.
Regulatory Requirements for Medical Device Recalls
Regulatory requirements for medical device recalls mandate prompt notification to authorities such as the FDA or EMA upon identifying risks to patient safety, with detailed documentation of the hazard and corrective measures. Product recalls involve removal or correction of defective devices from the market, while Field Safety Corrective Actions (FSCAs) typically address less critical issues through warnings or software updates without full withdrawal. Compliance with stringent guidelines ensures effective risk management, protecting public health and maintaining regulatory approval for continued device distribution.
FSCA Implementation: Steps and Best Practices
Effective FSCA implementation in medical devices involves a systematic approach starting with risk assessment, followed by timely notification to regulatory bodies and stakeholders. Critical steps include root cause analysis, development of corrective measures, and comprehensive documentation to ensure traceability and compliance. Best practices emphasize cross-functional collaboration, continuous monitoring of corrective action effectiveness, and transparent communication with healthcare providers to mitigate patient safety risks.
Risk Assessment: Recall vs FSCA Decision Criteria
Risk assessment for product recalls involves evaluating potential harm severity and probability, determining if device defects pose immediate danger to patient health or cause regulatory non-compliance. Field Safety Corrective Actions (FSCA) prioritize mitigating identified risks without full product withdrawal, focusing on targeted measures such as device modification, user instructions, or software updates. The decision criteria hinge on risk magnitude, occurrence likelihood, and the feasibility of correction without recalling, ensuring patient safety while minimizing disruption to medical device availability.
Manufacturer Responsibilities in Recall and FSCA
Manufacturers hold critical responsibilities in both Product Recall and Field Safety Corrective Action (FSCA) processes, ensuring patient safety and regulatory compliance. They must promptly identify, evaluate, and communicate potential risks associated with the medical device, coordinating with regulatory authorities to implement effective corrective measures, including product retrieval or modifications. Thorough documentation and transparent reporting are essential to mitigate hazards and maintain market trust during recalls and FSCAs.
Impact on Healthcare Providers and Patients
Product recalls in medical devices often result in immediate cessation of device use, directly affecting healthcare providers by disrupting clinical workflows and increasing the risk of treatment delays for patients. Field Safety Corrective Actions (FSCAs) typically involve less severe interventions such as software updates or device modifications, allowing continued device use while mitigating risks. The impact on patients varies, with recalls posing higher potential safety concerns and FSCAs focusing on risk management without significant interruption in patient care.
Communication Strategies During Recalls and FSCAs
Effective communication strategies during product recalls and Field Safety Corrective Actions (FSCAs) are critical to ensure timely notification of healthcare professionals and patients about potential risks associated with medical devices. Clear, precise information is disseminated through multiple channels including regulatory bodies, manufacturers, distributors, and healthcare providers to minimize confusion and maintain trust. Documentation must comply with regulations such as FDA 21 CFR Part 820 and EU MDR 2017/745 to ensure traceability and accountability throughout the recall and FSCA processes.
Post-Market Surveillance and Corrective Measures
Product Recall involves removing or correcting a medical device that presents a risk to health, while Field Safety Corrective Action (FSCA) includes modifications or warnings to reduce risk without full withdrawal. Effective Post-Market Surveillance (PMS) relies on continuous monitoring and reporting of device performance, adverse events, and user feedback to identify potential safety issues. Corrective measures through FSCA or recall are essential to mitigate identified risks, ensure regulatory compliance, and protect patient safety.
Product Recall vs Field Safety Corrective Action Infographic
 productdif.com
productdif.com