A Clinical Evaluation Report (CER) systematically assesses clinical data to demonstrate the safety and performance of a medical device before market approval, ensuring regulatory compliance. Post-market surveillance (PMS) continuously monitors the device's safety and effectiveness after commercialization through real-world data collection. Both CER and PMS are critical components of the device lifecycle, enabling manufacturers to identify risks and implement corrective actions to maintain patient safety.
Table of Comparison
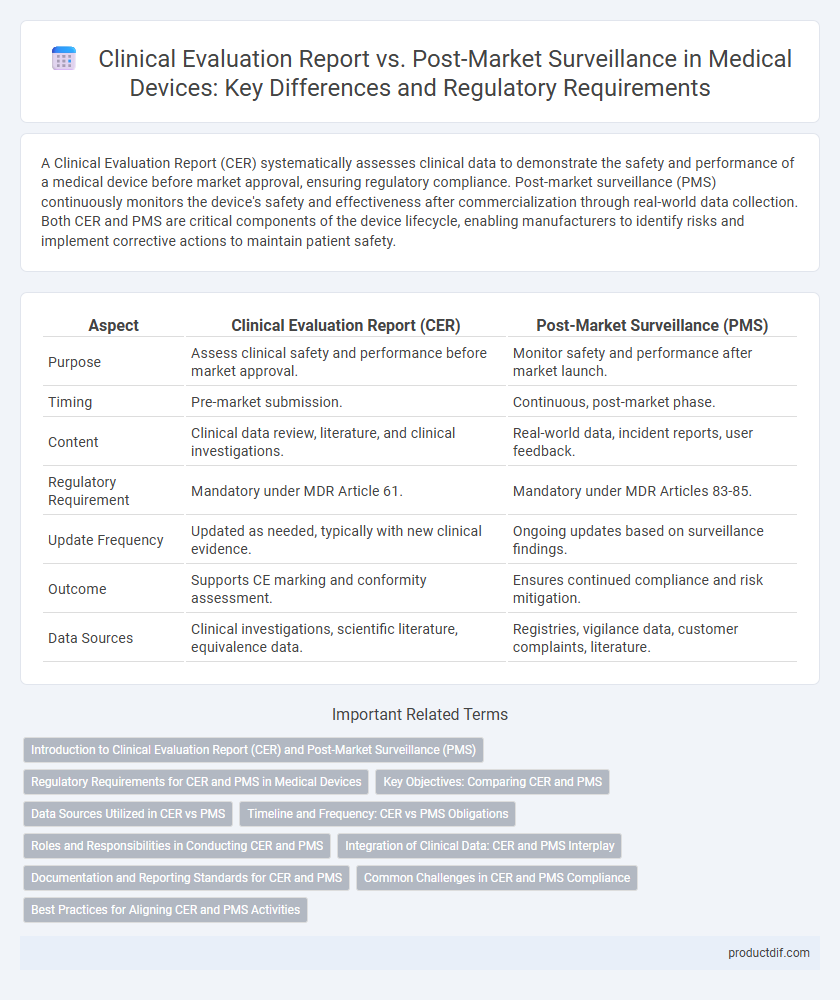
| Aspect | Clinical Evaluation Report (CER) | Post-Market Surveillance (PMS) |
|---|---|---|
| Purpose | Assess clinical safety and performance before market approval. | Monitor safety and performance after market launch. |
| Timing | Pre-market submission. | Continuous, post-market phase. |
| Content | Clinical data review, literature, and clinical investigations. | Real-world data, incident reports, user feedback. |
| Regulatory Requirement | Mandatory under MDR Article 61. | Mandatory under MDR Articles 83-85. |
| Update Frequency | Updated as needed, typically with new clinical evidence. | Ongoing updates based on surveillance findings. |
| Outcome | Supports CE marking and conformity assessment. | Ensures continued compliance and risk mitigation. |
| Data Sources | Clinical investigations, scientific literature, equivalence data. | Registries, vigilance data, customer complaints, literature. |
Introduction to Clinical Evaluation Report (CER) and Post-Market Surveillance (PMS)
The Clinical Evaluation Report (CER) systematically assesses clinical data to demonstrate a medical device's safety and performance before market approval. Post-Market Surveillance (PMS) continuously monitors the device's real-world safety and effectiveness after it enters the market, identifying potential risks and compliance issues. Both CER and PMS are essential components of regulatory compliance under medical device directives such as MDR 2017/745.
Regulatory Requirements for CER and PMS in Medical Devices
The Clinical Evaluation Report (CER) is a regulatory requirement that compiles clinical data to demonstrate a medical device's safety and performance before market approval, based on standards such as MDR 2017/745. Post-Market Surveillance (PMS) involves continuous data collection and analysis post-commercialization to monitor device performance and identify potential risks, ensuring compliance with regulatory mandates. Both CER and PMS are integral to maintaining device conformity, with CER supporting initial market access and PMS providing ongoing evidence for regulatory bodies like the FDA and European Notified Bodies.
Key Objectives: Comparing CER and PMS
Clinical Evaluation Reports (CER) primarily focus on the comprehensive assessment of clinical data to demonstrate a medical device's safety and performance before market approval. Post-Market Surveillance (PMS) emphasizes continuous monitoring and collection of real-world data to detect any emerging risks or device performance issues after commercialization. Key objectives of CER are to establish clinical compliance and risk-benefit analysis, while PMS aims to ensure ongoing device safety, effectiveness, and regulatory compliance through proactive vigilance.
Data Sources Utilized in CER vs PMS
Clinical evaluation reports (CER) primarily utilize pre-market clinical investigations, scientific literature, and clinical experience data to assess medical device safety and performance before market entry. Post-market surveillance (PMS) relies heavily on real-world data sources such as adverse event reports, registries, user feedback, and ongoing clinical follow-ups to monitor device performance after commercialization. Integrating data from both CER and PMS ensures comprehensive risk management and continuous improvement of medical devices.
Timeline and Frequency: CER vs PMS Obligations
The Clinical Evaluation Report (CER) is typically compiled before market entry, summarizing clinical evidence at a specific point with updates occurring periodically based on regulatory requirements or changes in device design. Post-Market Surveillance (PMS) obligations involve continuous and real-time monitoring of a medical device's safety and performance throughout its lifecycle, often requiring more frequent data collection and reporting to identify emerging risks. While CER updates are event-driven and less frequent, PMS activities maintain an ongoing obligation to gather and analyze post-market data regularly.
Roles and Responsibilities in Conducting CER and PMS
The Clinical Evaluation Report (CER) primarily involves assessing clinical data to demonstrate a medical device's safety and performance before market approval, requiring rigorous data analysis by clinical and regulatory experts. Post-Market Surveillance (PMS) focuses on ongoing data collection and risk management through active monitoring and feedback from real-world device use, led by quality assurance and compliance teams. Both CER and PMS demand collaboration between clinical specialists, regulatory affairs, and quality management to ensure continuous compliance with regulatory requirements and patient safety.
Integration of Clinical Data: CER and PMS Interplay
The Clinical Evaluation Report (CER) integrates pre-market and post-market clinical data to continuously assess the safety and performance of medical devices, ensuring regulatory compliance and patient safety. Post-Market Surveillance (PMS) collects real-world evidence that feeds back into the CER, enabling dynamic updates and risk management throughout the device lifecycle. Effective integration of CER and PMS data supports robust clinical evidence generation and facilitates proactive identification of emerging issues.
Documentation and Reporting Standards for CER and PMS
Clinical evaluation reports (CER) adhere to stringent documentation standards outlined in MEDDEV 2.7/1 revision 4, emphasizing comprehensive clinical data assessment and justification of safety and performance, while post-market surveillance (PMS) documentation follows ISO 13485 and MDR Annex III, focusing on continuous data collection, incident reporting, and trend analysis to ensure ongoing device compliance. CER documentation requires detailed synthesis of pre-market clinical evidence and risk-benefit analysis, whereas PMS reports involve systematic collection of real-world device performance metrics and corrective action records. Both documentation frameworks demand traceability, transparency, and alignment with regulatory authorities to maintain device market approval and patient safety.
Common Challenges in CER and PMS Compliance
Clinical Evaluation Reports (CER) and Post-Market Surveillance (PMS) share common challenges including data collection consistency, regulatory updates adaptation, and effectively integrating real-world evidence to demonstrate ongoing device safety and performance. Ensuring comprehensive and continuous risk management while maintaining traceability between clinical data and post-market findings is critical for compliance with MDR and FDA requirements. Inadequate documentation, gaps in clinical evidence, and delayed reporting often compromise CER and PMS effectiveness in safeguarding patient safety and regulatory adherence.
Best Practices for Aligning CER and PMS Activities
Clinical evaluation reports (CER) and post-market surveillance (PMS) activities must be closely integrated to ensure continuous safety and performance of medical devices, adhering to regulatory requirements like MDR 2017/745. Best practices include establishing a dynamic feedback loop where PMS data directly informs CER updates, enabling timely identification of potential risks and mitigation strategies. Leveraging real-world evidence and systematic data collection enhances traceability, supports compliance, and promotes proactive risk management throughout the device lifecycle.
Clinical evaluation report vs post-market surveillance Infographic
 productdif.com
productdif.com