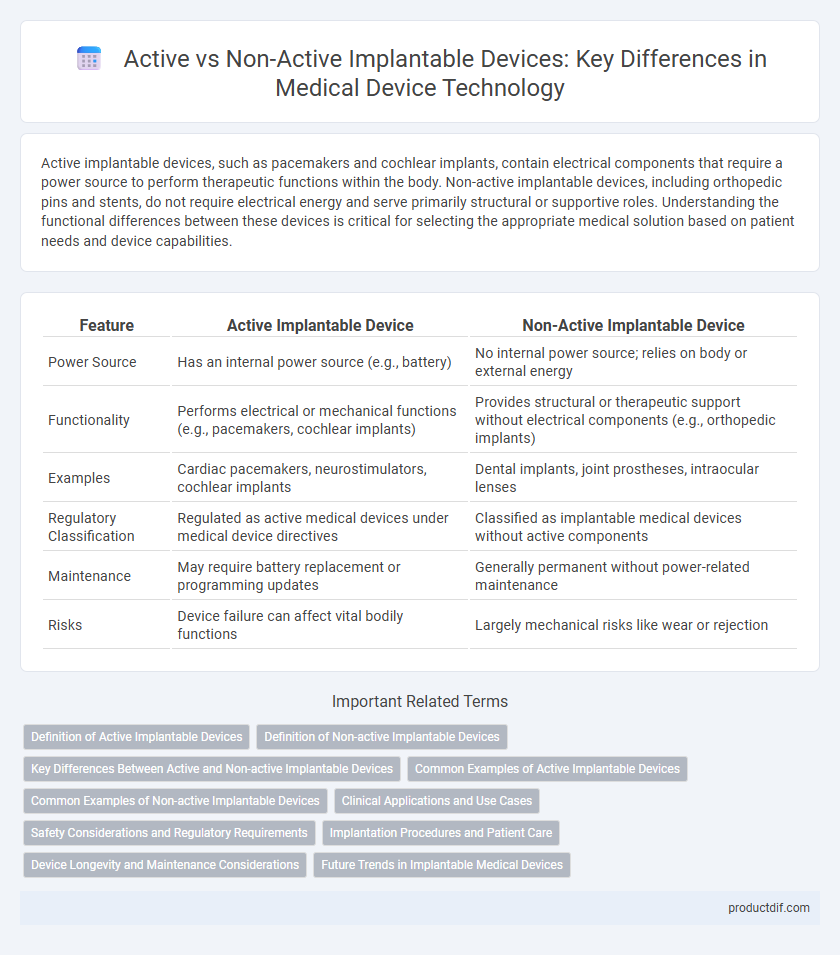
Active implantable devices, such as pacemakers and cochlear implants, contain electrical components that require a power source to perform therapeutic functions within the body. Non-active implantable devices, including orthopedic pins and stents, do not require electrical energy and serve primarily structural or supportive roles. Understanding the functional differences between these devices is critical for selecting the appropriate medical solution based on patient needs and device capabilities.
Table of Comparison
| Feature | Active Implantable Device | Non-Active Implantable Device |
|---|---|---|
| Power Source | Has an internal power source (e.g., battery) | No internal power source; relies on body or external energy |
| Functionality | Performs electrical or mechanical functions (e.g., pacemakers, cochlear implants) | Provides structural or therapeutic support without electrical components (e.g., orthopedic implants) |
| Examples | Cardiac pacemakers, neurostimulators, cochlear implants | Dental implants, joint prostheses, intraocular lenses |
| Regulatory Classification | Regulated as active medical devices under medical device directives | Classified as implantable medical devices without active components |
| Maintenance | May require battery replacement or programming updates | Generally permanent without power-related maintenance |
| Risks | Device failure can affect vital bodily functions | Largely mechanical risks like wear or rejection |
Definition of Active Implantable Devices
Active implantable devices are medical devices intended to be totally or partially introduced into the human body and rely on a power source, such as batteries or external power, to perform their functions. Examples include pacemakers, cochlear implants, and neurostimulators, which actively deliver electrical stimulation or therapeutic signals. These devices differ from non-active implantable devices, which do not require an external power source and include items like surgical meshes or artificial joints.
Definition of Non-active Implantable Devices
Non-active implantable devices are medical devices intended to be totally implanted in the human body without relying on any source of electrical power or energy. These devices function through passive mechanical or structural means, such as orthopedic implants, pacemaker leads, or intraocular lenses. Their primary role is to support, replace, or enhance physiological functions without electronic or battery-driven components.
Key Differences Between Active and Non-active Implantable Devices
Active implantable devices, such as pacemakers and cochlear implants, contain electrical components that require a power source to function and actively interact with body tissues. Non-active implantable devices, like orthopedic screws and breast implants, do not rely on electrical power and serve primarily structural or therapeutic roles without electronic activity. The key differences lie in energy dependency, functionality, and regulatory classifications, impacting design, safety requirements, and clinical applications.
Common Examples of Active Implantable Devices
Common examples of active implantable devices include pacemakers, cochlear implants, and neurostimulators, which rely on electrical energy to perform their intended medical functions. These devices often contain batteries and electronic circuits that actively regulate physiological processes or restore sensory functions. In contrast, non-active implantable devices such as orthopedic implants or surgical meshes do not require electrical power and primarily provide structural support or tissue reinforcement.
Common Examples of Non-active Implantable Devices
Non-active implantable devices include common examples such as orthopedic screws, plates, stents, and pacemaker leads that do not require an external power source to function. These devices are typically made from biocompatible materials like titanium, stainless steel, or medical-grade polymers to ensure durability and integration with body tissues. Unlike active implantable devices, non-active implants serve structural or supportive roles without electronic or battery-powered components.
Clinical Applications and Use Cases
Active implantable devices such as pacemakers and cochlear implants deliver electrical stimulation to regulate bodily functions or restore sensory capabilities, making them essential in cardiac rhythm management and hearing restoration. Non-active implantable devices like orthopedic screws, plates, and intraocular lenses provide structural support or replace damaged tissues without electrical components, primarily used in fracture fixation and vision correction. Clinical applications of active devices focus on dynamic physiological intervention, while non-active devices are crucial for mechanical support and anatomical restoration.
Safety Considerations and Regulatory Requirements
Active implantable devices, such as pacemakers, require rigorous safety considerations including electromagnetic compatibility, battery longevity, and software reliability to prevent malfunction and ensure patient safety. Non-active implantable devices, like orthopedic implants, primarily focus on biocompatibility, mechanical integrity, and risk of infection to maintain long-term safety and functionality. Regulatory requirements for active devices involve stringent pre-market clinical evaluations, post-market surveillance, and compliance with ISO 14708 standards, while non-active devices adhere to standards such as ISO 10993 for biological evaluation and FDA 510(k) pathways emphasizing material safety and structural performance.
Implantation Procedures and Patient Care
Active implantable devices, such as pacemakers and cochlear implants, require precise implantation procedures involving electrical connection to tissues, demanding specialized surgical skills and postoperative device programming for patient safety and functionality. Non-active implantable devices, like pacemaker leads or joint prostheses, focus primarily on biocompatible material placement without electronic components, resulting in less complex surgical steps but requiring vigilant monitoring for mechanical integrity and tissue integration. Patient care for active implants emphasizes device management through regular follow-ups and adjustment, while non-active implants prioritize infection control and physical rehabilitation to ensure optimal long-term outcomes.
Device Longevity and Maintenance Considerations
Active implantable devices, such as pacemakers and neurostimulators, require periodic battery replacements or recharging to maintain optimal functionality, impacting their overall device longevity. Non-active implantable devices, like orthopedic implants or dental implants, generally have longer lifespans with minimal maintenance, relying primarily on biocompatibility and mechanical durability. Maintenance considerations for active devices include scheduled clinical evaluations and software updates, whereas non-active devices focus on monitoring for wear or biological reactions.
Future Trends in Implantable Medical Devices
Future trends in implantable medical devices emphasize advancements in active implantable devices, including increased integration of smart sensors and wireless communication technology to enable real-time monitoring and personalized therapy. Non-active implantable devices are evolving with biocompatible materials and improved design for enhanced durability and patient comfort. Emerging innovations focus on hybrid systems that combine active electronics with passive structures to optimize functionality and long-term patient outcomes.
Active implantable device vs Non-active implantable device Infographic
 productdif.com
productdif.com