The Design History File (DHF) documents the design development process of a medical device, capturing design inputs, outputs, verification, and validation activities to ensure compliance with regulatory requirements. The Device Master Record (DMR) contains detailed instructions, specifications, and procedures necessary for manufacturing the device consistently and within quality standards. Maintaining both DHF and DMR is critical for demonstrating product traceability and regulatory adherence throughout the device lifecycle.
Table of Comparison
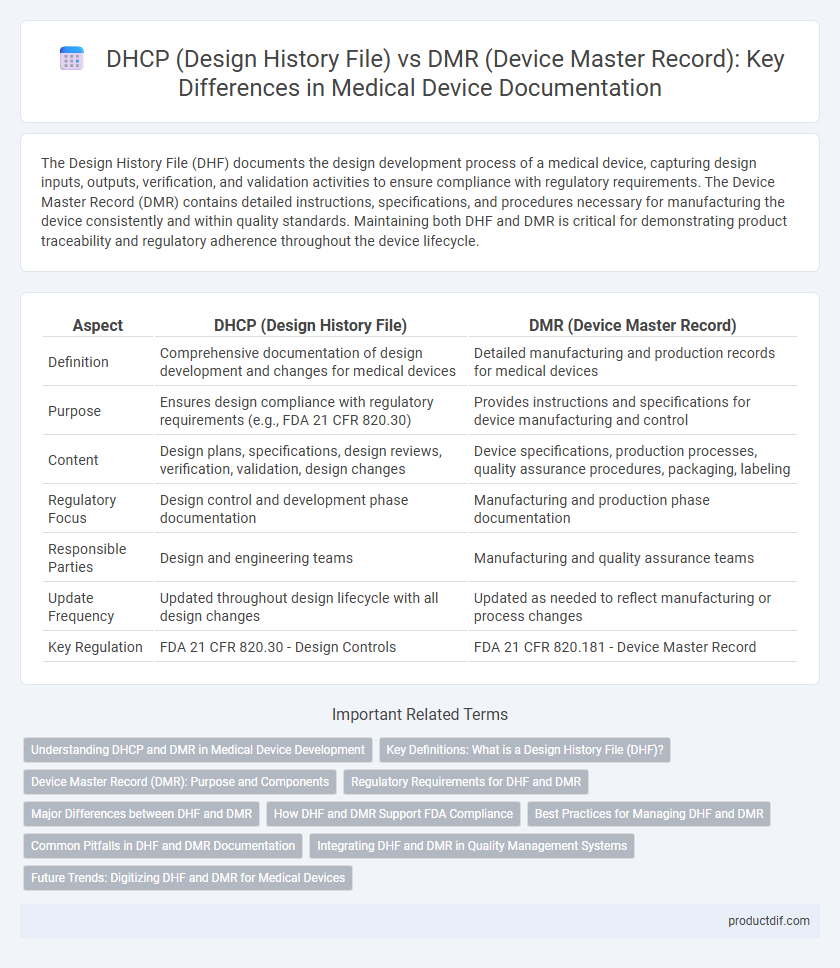
| Aspect | DHCP (Design History File) | DMR (Device Master Record) |
|---|---|---|
| Definition | Comprehensive documentation of design development and changes for medical devices | Detailed manufacturing and production records for medical devices |
| Purpose | Ensures design compliance with regulatory requirements (e.g., FDA 21 CFR 820.30) | Provides instructions and specifications for device manufacturing and control |
| Content | Design plans, specifications, design reviews, verification, validation, design changes | Device specifications, production processes, quality assurance procedures, packaging, labeling |
| Regulatory Focus | Design control and development phase documentation | Manufacturing and production phase documentation |
| Responsible Parties | Design and engineering teams | Manufacturing and quality assurance teams |
| Update Frequency | Updated throughout design lifecycle with all design changes | Updated as needed to reflect manufacturing or process changes |
| Key Regulation | FDA 21 CFR 820.30 - Design Controls | FDA 21 CFR 820.181 - Device Master Record |
Understanding DHCP and DMR in Medical Device Development
The Design History File (DHCP) documents the comprehensive development process of a medical device, including design inputs, outputs, verification, and validation activities, ensuring traceability from concept to production. The Device Master Record (DMR) contains detailed instructions, specifications, and procedures for manufacturing, packaging, labeling, and installing the finished device, serving as the authoritative production reference. Understanding the distinction between DHCP and DMR is critical for regulatory compliance and effective lifecycle management in medical device development.
Key Definitions: What is a Design History File (DHF)?
A Design History File (DHF) is a comprehensive record that documents the design development process of a medical device, including design inputs, outputs, verification, validation, and design changes. It ensures traceability and regulatory compliance by detailing how design requirements are met throughout the product lifecycle. Unlike the Device Master Record (DMR), which contains the manufacturing specifications and procedures, the DHF focuses exclusively on the design and development documentation.
Device Master Record (DMR): Purpose and Components
The Device Master Record (DMR) serves as a comprehensive compilation of all instructions, drawings, and specifications essential for manufacturing a medical device. It includes detailed component specifications, production processes, quality assurance protocols, and packaging requirements, ensuring consistent product quality. By maintaining an accurate DMR, manufacturers achieve regulatory compliance and facilitate effective device reproduction and traceability.
Regulatory Requirements for DHF and DMR
The Design History File (DHF) must comprehensively document the design development process, demonstrating compliance with FDA 21 CFR 820.30 and ISO 13485 regulations to ensure traceability and design control adherence. The Device Master Record (DMR) contains detailed specifications, production processes, quality assurance procedures, and acceptance criteria, meeting regulatory requirements outlined by FDA 21 CFR 820.181 for manufacturing consistency and product realization. Both DHF and DMR are essential for FDA audits and CE marking, supporting regulatory submissions and post-market surveillance through meticulous documentation.
Major Differences between DHF and DMR
The Design History File (DHF) documents the design process, specifications, and development activities of a medical device, ensuring compliance with regulatory standards such as FDA 21 CFR Part 820. The Device Master Record (DMR) contains the detailed manufacturing instructions, including production processes, quality assurance protocols, and assembly procedures required to produce the device consistently. Major differences between DHF and DMR lie in their purposes: DHF focuses on design documentation and engineering controls, while DMR centers on manufacturing and production details to maintain product quality.
How DHF and DMR Support FDA Compliance
The Design History File (DHF) documents the design evolution and verification processes, ensuring traceability and adherence to FDA's design control requirements under 21 CFR 820.30. The Device Master Record (DMR) contains detailed manufacturing specifications, procedures, and quality assurance protocols critical for FDA compliance in production consistency as mandated by 21 CFR 820.181. Together, DHF and DMR establish comprehensive FDA regulatory compliance by linking design validation with controlled manufacturing execution.
Best Practices for Managing DHF and DMR
Effective management of the Design History File (DHF) and Device Master Record (DMR) is crucial for medical device regulatory compliance and product quality. Best practices include maintaining comprehensive, organized, and audit-ready documentation that clearly traces design controls throughout the product lifecycle in the DHF, while ensuring the DMR accurately reflects the final approved device specifications and manufacturing processes. Implementing robust version control systems and regular cross-functional reviews facilitates seamless updates and alignment between the DHF and DMR, minimizing risks of discrepancies and regulatory nonconformities.
Common Pitfalls in DHF and DMR Documentation
Common pitfalls in Design History File (DHF) and Device Master Record (DMR) documentation include incomplete traceability between design inputs, outputs, and verification activities, leading to regulatory non-compliance. Inadequate version control and failure to update records during design changes cause discrepancies that complicate device validation and audit processes. Poor organization and lack of clear documentation protocols result in missing critical design controls and manufacturing instructions, increasing risk of device recalls and FDA inspection findings.
Integrating DHF and DMR in Quality Management Systems
Integrating the Design History File (DHF) and Device Master Record (DMR) within Quality Management Systems (QMS) enhances traceability and compliance throughout the medical device lifecycle. DHF documents all design controls and development activities, while DMR contains the complete specifications and manufacturing instructions for the finished device. Synchronizing these records ensures seamless transition from design to production, improving regulatory readiness under FDA 21 CFR Part 820 requirements.
Future Trends: Digitizing DHF and DMR for Medical Devices
Future trends in medical device management emphasize the digitization of Design History Files (DHF) and Device Master Records (DMR) to enhance traceability, compliance, and efficiency. Implementing cloud-based platforms and blockchain technology facilitates real-time updates and secure, immutable documentation critical for regulatory audits. Advanced AI-driven analytics integrated within digital DHF and DMR systems enable predictive quality control and streamline product lifecycle management.
DHCP (design history file) vs DMR (device master record) Infographic
 productdif.com
productdif.com