Corrective action addresses and resolves issues after a nonconformance or defect is identified in medical devices, ensuring the problem does not recur. Preventive action involves proactive measures to identify potential risks and implement solutions before defects or regulatory noncompliance occur. Both actions are essential components of quality management systems for maintaining product safety and regulatory compliance in the medical device industry.
Table of Comparison
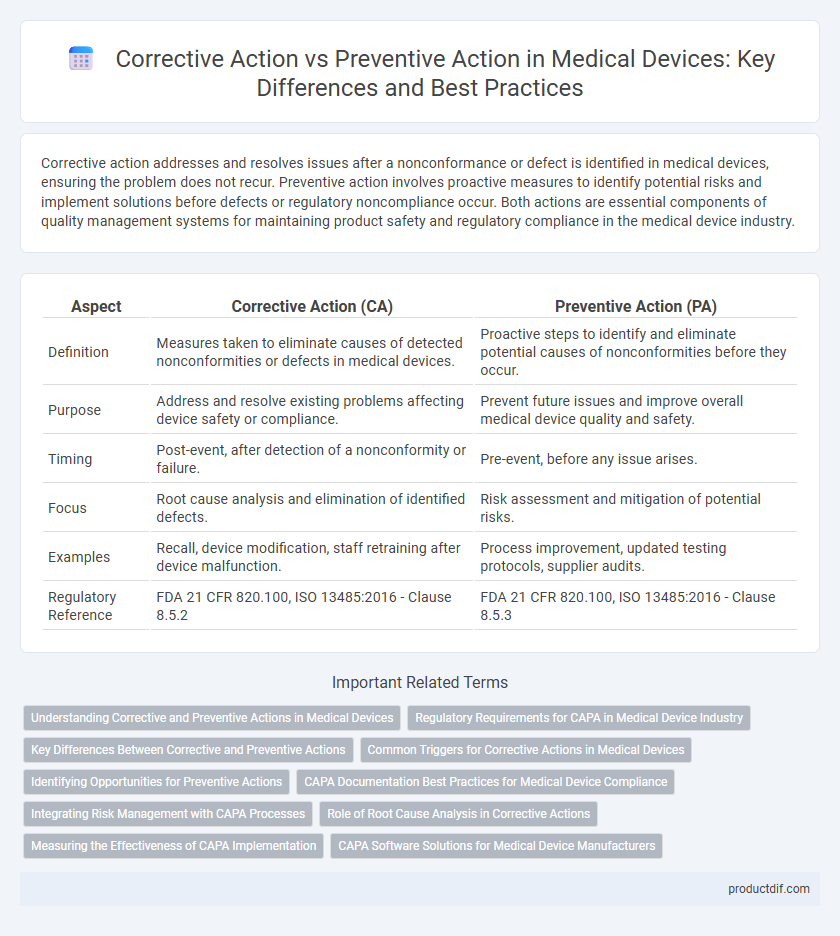
| Aspect | Corrective Action (CA) | Preventive Action (PA) |
|---|---|---|
| Definition | Measures taken to eliminate causes of detected nonconformities or defects in medical devices. | Proactive steps to identify and eliminate potential causes of nonconformities before they occur. |
| Purpose | Address and resolve existing problems affecting device safety or compliance. | Prevent future issues and improve overall medical device quality and safety. |
| Timing | Post-event, after detection of a nonconformity or failure. | Pre-event, before any issue arises. |
| Focus | Root cause analysis and elimination of identified defects. | Risk assessment and mitigation of potential risks. |
| Examples | Recall, device modification, staff retraining after device malfunction. | Process improvement, updated testing protocols, supplier audits. |
| Regulatory Reference | FDA 21 CFR 820.100, ISO 13485:2016 - Clause 8.5.2 | FDA 21 CFR 820.100, ISO 13485:2016 - Clause 8.5.3 |
Understanding Corrective and Preventive Actions in Medical Devices
Corrective Actions in medical devices address identified nonconformities or defects by implementing immediate fixes to prevent recurrence, focusing on resolving current issues such as product failures or adverse events. Preventive Actions aim to identify potential risks and implement controls before defects occur, ensuring compliance with regulatory standards like ISO 13485 and FDA 21 CFR Part 820. Effective understanding of CAPA (Corrective and Preventive Actions) processes improves device safety, regulatory compliance, and continuous quality enhancement in medical device manufacturing.
Regulatory Requirements for CAPA in Medical Device Industry
Regulatory requirements for Corrective Action and Preventive Action (CAPA) in the medical device industry mandate strict adherence to guidelines established by regulatory bodies such as the FDA and ISO 13485. CAPA systems must include procedures for identifying nonconformities, investigating root causes, implementing corrective measures, and verifying their effectiveness to ensure product safety and compliance. Documentation and traceability of CAPA activities are critical to meet regulatory audits and maintain market approval for medical devices.
Key Differences Between Corrective and Preventive Actions
Corrective actions in medical device manufacturing address detected nonconformities or failures by eliminating causes to prevent recurrence, focusing on reactive problem-solving after an issue arises. Preventive actions proactively identify and mitigate potential risks before defects occur, aiming to improve processes and prevent future problems. The key difference lies in their timing and focus: corrective actions respond to actual issues, while preventive actions target potential risks to enhance overall quality and safety.
Common Triggers for Corrective Actions in Medical Devices
Common triggers for corrective actions in medical devices include incidents of device malfunctions, complaints reporting safety or performance issues, and deviations identified during quality audits or inspections. Nonconformities such as software failures, packaging defects, and labeling errors also frequently prompt corrective action processes. Data from post-market surveillance and adverse event reports provide critical insight leading to timely corrective interventions.
Identifying Opportunities for Preventive Actions
Identifying opportunities for preventive actions in medical device manufacturing involves analyzing trends from nonconformances, customer complaints, and audit findings to proactively address potential risks. Implementing robust risk management processes such as Failure Mode and Effects Analysis (FMEA) helps detect vulnerabilities before issues arise. This strategic approach minimizes product defects and regulatory non-compliance, enhancing overall device safety and performance.
CAPA Documentation Best Practices for Medical Device Compliance
Effective CAPA documentation for medical device compliance requires detailed records of both Corrective Action and Preventive Action processes, emphasizing root cause analysis and risk assessment. Accurate documentation must include clear objectives, implementation steps, verification results, and timelines to satisfy FDA and ISO 13485 standards. Consistent CAPA record-keeping enhances traceability, supports audits, and ensures continuous improvement in device quality and patient safety.
Integrating Risk Management with CAPA Processes
Integrating risk management with Corrective and Preventive Action (CAPA) processes enhances the effectiveness of medical device quality systems by systematically identifying, evaluating, and mitigating potential and actual risks. By leveraging risk-based approaches, manufacturers can prioritize CAPA activities according to severity and probability, ensuring robust compliance with regulatory standards such as FDA 21 CFR Part 820 and ISO 13485. This integration facilitates continuous improvement, reduces product failures, and strengthens patient safety through proactive identification and resolution of device-related hazards.
Role of Root Cause Analysis in Corrective Actions
Root Cause Analysis (RCA) plays a crucial role in Corrective Actions within medical device quality management by identifying the fundamental cause of nonconformities or failures. Addressing the root cause enables targeted corrective measures that eliminate recurrence, ensuring compliance with FDA and ISO 13485 standards. Effective RCA enhances product safety, reduces risk, and improves overall device reliability by preventing repeat defects.
Measuring the Effectiveness of CAPA Implementation
Measuring the effectiveness of CAPA implementation in medical devices involves evaluating key performance indicators such as the reduction in nonconformities, trend analysis of product quality data, and audit results. Corrective actions focus on addressing existing problems, while preventive actions aim to eliminate potential risks before occurrence. Effective CAPA implementation ensures continuous improvement in compliance with FDA regulations and ISO 13485 standards.
CAPA Software Solutions for Medical Device Manufacturers
CAPA software solutions for medical device manufacturers streamline corrective action and preventive action processes by enabling efficient identification, documentation, and resolution of nonconformities and potential risks. These platforms integrate real-time data analytics and automated workflows to enhance compliance with regulatory standards such as FDA 21 CFR Part 820 and ISO 13485. Implementing CAPA software reduces response times, improves quality management system effectiveness, and supports continuous improvement in medical device production and post-market surveillance.
Corrective Action vs Preventive Action Infographic
 productdif.com
productdif.com