A 510(k) submission demonstrates that a medical device is substantially equivalent to an existing legally marketed device, often requiring less time and lower costs compared to a PMA submission. Premarket Approval (PMA) is a more rigorous process reserved for high-risk devices, requiring extensive clinical data to prove safety and effectiveness. Manufacturers must carefully assess device classification and intended use to determine the appropriate FDA regulatory pathway for market entry.
Table of Comparison
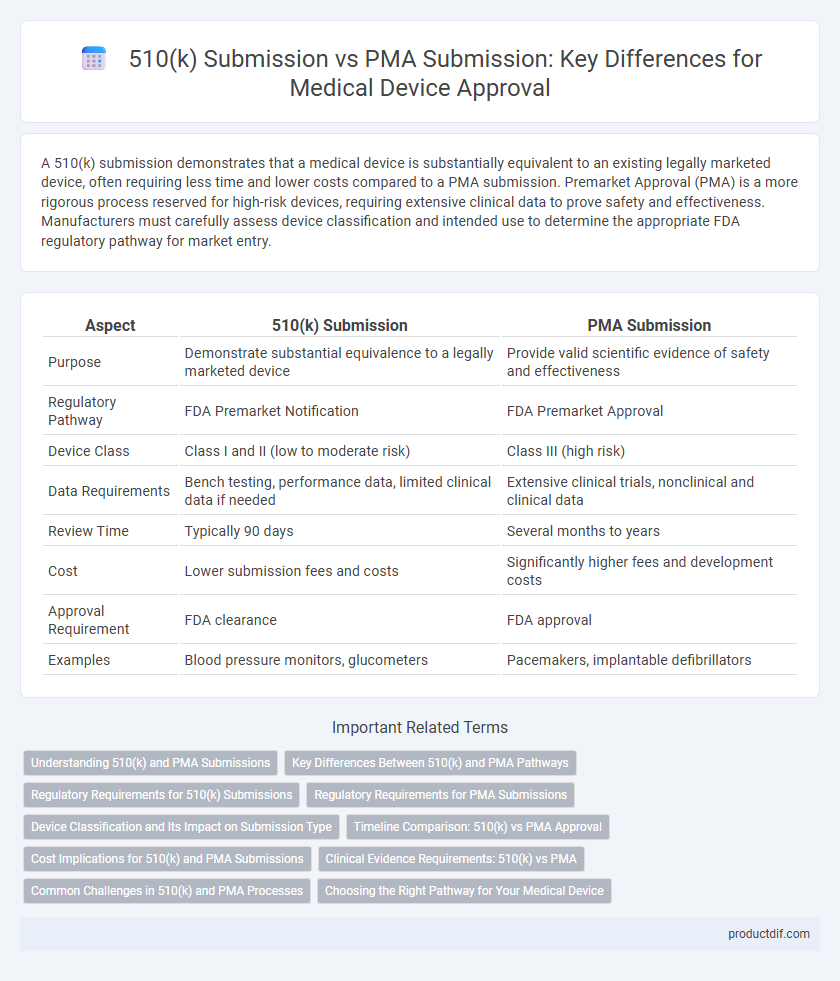
| Aspect | 510(k) Submission | PMA Submission |
|---|---|---|
| Purpose | Demonstrate substantial equivalence to a legally marketed device | Provide valid scientific evidence of safety and effectiveness |
| Regulatory Pathway | FDA Premarket Notification | FDA Premarket Approval |
| Device Class | Class I and II (low to moderate risk) | Class III (high risk) |
| Data Requirements | Bench testing, performance data, limited clinical data if needed | Extensive clinical trials, nonclinical and clinical data |
| Review Time | Typically 90 days | Several months to years |
| Cost | Lower submission fees and costs | Significantly higher fees and development costs |
| Approval Requirement | FDA clearance | FDA approval |
| Examples | Blood pressure monitors, glucometers | Pacemakers, implantable defibrillators |
Understanding 510(k) and PMA Submissions
510(k) submissions demonstrate that a new medical device is substantially equivalent to a legally marketed predicate device, streamlining FDA clearance for moderate-risk devices. Premarket Approval (PMA) submissions require extensive clinical data and rigorous scientific evidence to prove safety and effectiveness, essential for high-risk medical devices. Understanding the regulatory distinctions between 510(k) and PMA processes helps manufacturers navigate FDA requirements and select the appropriate approval pathway.
Key Differences Between 510(k) and PMA Pathways
510(k) submission primarily targets moderate-risk medical devices by demonstrating substantial equivalence to a legally marketed predicate device, enabling a faster clearance process typically within 90 days. PMA submission is required for high-risk devices, involving a rigorous scientific review of safety and effectiveness supported by clinical data, often taking several months to years for FDA approval. The key differences include the level of evidence required, regulatory scrutiny, and duration of the approval process, with PMA being more stringent and comprehensive than the 510(k) pathway.
Regulatory Requirements for 510(k) Submissions
The 510(k) submission requires demonstrating that a medical device is substantially equivalent to a legally marketed predicate device, focusing on safety and effectiveness without extensive clinical data. Key regulatory requirements for 510(k) include accurate device description, labeling, performance testing, and risk analysis to meet FDA standards. This pathway is typically faster and less costly compared to Premarket Approval (PMA), which mandates comprehensive clinical trials and detailed scientific evidence.
Regulatory Requirements for PMA Submissions
PMA submissions require extensive clinical data demonstrating a medical device's safety and effectiveness, significantly exceeding the data required for 510(k) submissions. The FDA mandates detailed manufacturing information, preclinical and clinical study results, and labeling that ensures proper device use for PMA approval. Rigorous post-market surveillance and adherence to Quality System Regulations (QSR) are essential components of PMA regulatory requirements to maintain compliance and patient safety.
Device Classification and Its Impact on Submission Type
Medical devices are classified by the FDA into Class I, II, or III based on risk, directly influencing the submission type for market clearance. Class I and most Class II devices typically require a 510(k) submission, demonstrating substantial equivalence to a legally marketed predicate device. Class III devices, which pose the highest risk, require a Premarket Approval (PMA) submission, involving rigorous scientific review to ensure safety and effectiveness before marketing.
Timeline Comparison: 510(k) vs PMA Approval
The 510(k) submission process for medical devices typically takes around 90 days for FDA clearance, making it a faster pathway compared to Premarket Approval (PMA), which can take anywhere from 180 days to several months or even years depending on the device complexity. PMA requires extensive clinical data and rigorous review, contributing to longer approval timelines compared to the substantial equivalence demonstration required in 510(k) submissions. Manufacturers should consider the time-sensitive nature of their product launch when choosing between 510(k) clearance and PMA approval routes.
Cost Implications for 510(k) and PMA Submissions
510(k) submissions typically incur lower costs, ranging from $5,000 to $40,000, due to streamlined review processes and reliance on substantial equivalence to predicate devices. In contrast, Premarket Approval (PMA) submissions often exceed $250,000, reflecting extensive clinical data requirements and rigorous FDA evaluation. Device manufacturers must consider these cost disparities when determining regulatory pathways for medical device market entry.
Clinical Evidence Requirements: 510(k) vs PMA
The clinical evidence requirements for a 510(k) submission typically involve demonstrating substantial equivalence to a predicate device, often relying on bench testing and limited clinical data, whereas a PMA submission demands extensive clinical trials providing robust safety and effectiveness data. PMA submissions require rigorous, statistically significant clinical evidence, including multicenter studies and patient outcomes, due to the higher risk classification of the device. The FDA scrutinizes PMA clinical data in greater detail compared to 510(k) to ensure compliance with stringent safety standards for novel or high-risk medical devices.
Common Challenges in 510(k) and PMA Processes
510(k) submission challenges often include demonstrating substantial equivalence to a predicate device, navigating ambiguous FDA guidance, and managing insufficient clinical data. PMA submissions require rigorous scientific evidence, extensive clinical trials, and lengthy FDA review timelines, leading to higher costs and resource demands. Both pathways face complexities in regulatory documentation, evolving FDA requirements, and potential delays due to incomplete or inconsistent data.
Choosing the Right Pathway for Your Medical Device
Selecting the appropriate regulatory pathway for your medical device depends on its risk classification and intended use, with 510(k) submission typically required for moderate-risk devices demonstrating substantial equivalence to a legally marketed device. Premarket Approval (PMA) is mandatory for high-risk devices and requires comprehensive scientific evidence to ensure safety and effectiveness. Understanding the differences between 510(k) and PMA submissions enables manufacturers to streamline the approval process and align with FDA regulations efficiently.
510(k) submission vs PMA submission Infographic
 productdif.com
productdif.com