Home-use diagnostic devices empower patients to monitor health conditions conveniently and regularly within their own environment, enhancing early detection and ongoing management. Point-of-care diagnostic tools, typically used by healthcare professionals in clinical or emergency settings, provide rapid and accurate results to facilitate immediate medical decisions. Both technologies improve patient outcomes by enabling timely interventions, but differ in accessibility, user expertise, and application scenarios.
Table of Comparison
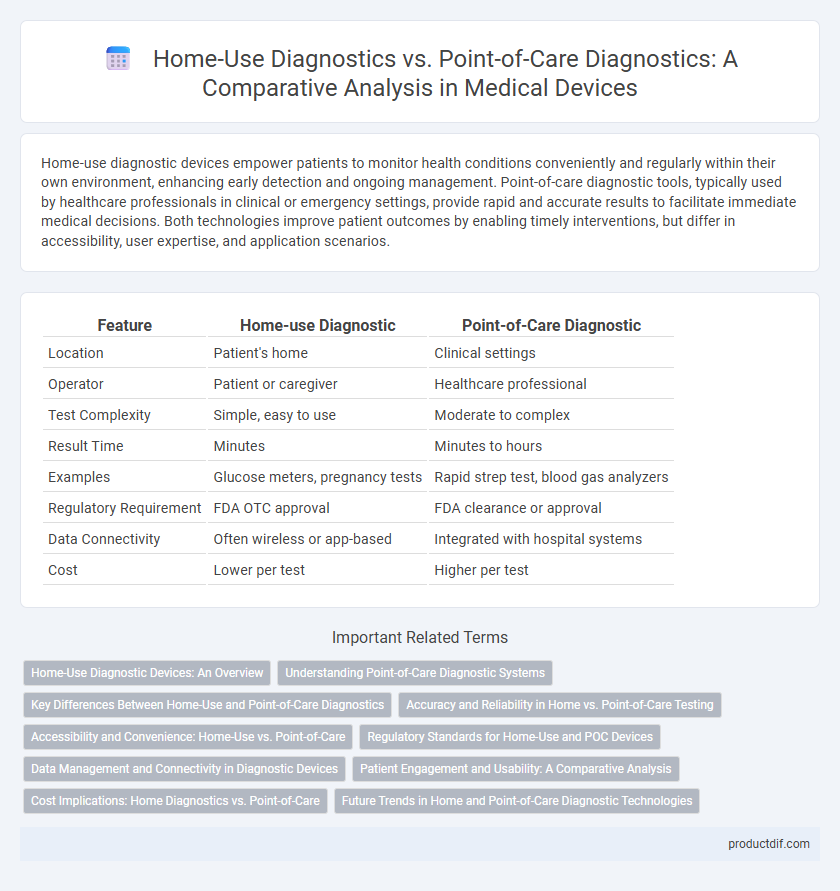
| Feature | Home-use Diagnostic | Point-of-Care Diagnostic |
|---|---|---|
| Location | Patient's home | Clinical settings |
| Operator | Patient or caregiver | Healthcare professional |
| Test Complexity | Simple, easy to use | Moderate to complex |
| Result Time | Minutes | Minutes to hours |
| Examples | Glucose meters, pregnancy tests | Rapid strep test, blood gas analyzers |
| Regulatory Requirement | FDA OTC approval | FDA clearance or approval |
| Data Connectivity | Often wireless or app-based | Integrated with hospital systems |
| Cost | Lower per test | Higher per test |
Home-Use Diagnostic Devices: An Overview
Home-use diagnostic devices enable patients to monitor health conditions independently, offering convenience and timely detection of abnormalities. These devices typically include blood glucose meters, pregnancy tests, and home cholesterol monitors, designed for ease of use and accurate results outside clinical environments. Advances in technology have enhanced the reliability and connectivity of home-use diagnostics, integrating with mobile apps for real-time health management.
Understanding Point-of-Care Diagnostic Systems
Point-of-care diagnostic systems enable rapid testing and immediate results at or near the site of patient care, improving clinical decision-making and treatment outcomes. These systems integrate advanced biosensors, microfluidics, and connected digital platforms to provide accurate diagnostics outside traditional laboratory settings. Home-use diagnostics, while convenient for patient self-monitoring, typically offer less comprehensive data and rely heavily on user interpretation compared to point-of-care systems operated by healthcare professionals.
Key Differences Between Home-Use and Point-of-Care Diagnostics
Home-use diagnostic devices are designed for patient-operated testing in non-clinical settings, emphasizing ease of use, portability, and rapid results without professional assistance. Point-of-care diagnostics, while also providing quick results, are intended for use by healthcare professionals within clinical environments, offering higher accuracy and comprehensive data for immediate medical decisions. Key differences include the level of user expertise required, complexity of technology, and the clinical context in which the device is deployed.
Accuracy and Reliability in Home vs. Point-of-Care Testing
Home-use diagnostic devices prioritize user-friendly interfaces and ease of operation, but their accuracy and reliability can be affected by user error and less controlled environments. Point-of-care diagnostic tests, conducted by trained healthcare professionals, generally demonstrate higher precision and consistent reliability due to standardized procedures and controlled settings. Advances in sensor technology and digital connectivity are narrowing the accuracy gap between home-use and point-of-care diagnostics, enabling more reliable patient self-monitoring.
Accessibility and Convenience: Home-Use vs. Point-of-Care
Home-use diagnostic devices offer unparalleled accessibility by allowing patients to conduct tests independently in their own environment, eliminating the need for travel or scheduling appointments. Point-of-care diagnostics provide convenience through rapid results at or near the site of patient care, facilitating immediate clinical decision-making by healthcare professionals. Both modalities enhance healthcare delivery, with home-use diagnostics promoting patient autonomy and point-of-care tools ensuring timely medical intervention.
Regulatory Standards for Home-Use and POC Devices
Home-use diagnostic devices must comply with FDA Class II or Class III regulations, emphasizing user safety, ease of operation, and accuracy for non-professional settings. Point-of-care (POC) diagnostic devices are often subject to CLIA waivers to ensure quality and reliability under healthcare provider supervision. Both categories require rigorous validation and labeling standards to meet regulatory requirements specific to their intended use environments.
Data Management and Connectivity in Diagnostic Devices
Home-use diagnostic devices prioritize user-friendly data management with secure cloud connectivity for remote monitoring and seamless integration with personal health records. Point-of-care diagnostic devices emphasize real-time data transfer and interoperability within clinical information systems to support immediate clinical decision-making. Advanced connectivity features like Bluetooth, Wi-Fi, and IoT protocols enhance data accuracy and accessibility across both settings, improving patient outcomes and workflow efficiency.
Patient Engagement and Usability: A Comparative Analysis
Home-use diagnostic devices empower patients by offering convenience and fostering proactive health management through intuitive interfaces and real-time feedback, enhancing patient engagement. Point-of-care diagnostics, typically used in clinical settings, deliver rapid results with professional oversight, which supports accurate interpretation but may limit patient autonomy. Analyzing usability reveals that home-use diagnostics prioritize user-friendly designs suitable for non-experts, whereas point-of-care devices emphasize clinical precision and integration within healthcare workflows.
Cost Implications: Home Diagnostics vs. Point-of-Care
Home-use diagnostic devices typically offer lower upfront costs due to mass production and simplified technology, making them cost-effective for frequent, routine monitoring. Point-of-care diagnostics often entail higher expenses related to specialized equipment and trained personnel, leading to increased operational costs in clinical settings. Budget decisions must weigh the affordability and accessibility of home devices against the comprehensive capabilities and faster results provided by point-of-care testing.
Future Trends in Home and Point-of-Care Diagnostic Technologies
Home-use diagnostic devices are increasingly integrating advanced AI algorithms and IoT connectivity to provide real-time health monitoring and personalized insights, enhancing patient empowerment and chronic disease management. Point-of-care diagnostic technologies are evolving with miniaturized biosensors and rapid multiplex assays, enabling quicker and more accurate onsite clinical decisions. Future trends emphasize seamless data integration with electronic health records (EHRs) and the development of non-invasive sampling techniques to improve accessibility and user compliance.
Home-use Diagnostic vs Point-of-Care Diagnostic Infographic
 productdif.com
productdif.com