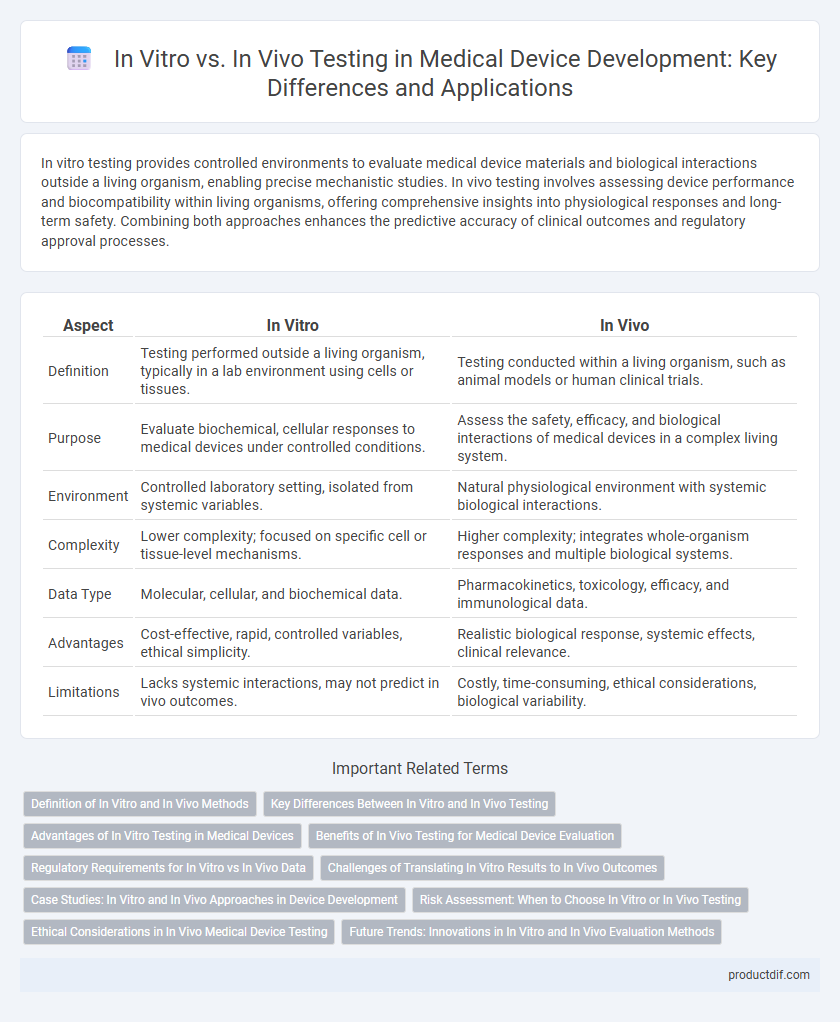
In vitro testing provides controlled environments to evaluate medical device materials and biological interactions outside a living organism, enabling precise mechanistic studies. In vivo testing involves assessing device performance and biocompatibility within living organisms, offering comprehensive insights into physiological responses and long-term safety. Combining both approaches enhances the predictive accuracy of clinical outcomes and regulatory approval processes.
Table of Comparison
| Aspect | In Vitro | In Vivo |
|---|---|---|
| Definition | Testing performed outside a living organism, typically in a lab environment using cells or tissues. | Testing conducted within a living organism, such as animal models or human clinical trials. |
| Purpose | Evaluate biochemical, cellular responses to medical devices under controlled conditions. | Assess the safety, efficacy, and biological interactions of medical devices in a complex living system. |
| Environment | Controlled laboratory setting, isolated from systemic variables. | Natural physiological environment with systemic biological interactions. |
| Complexity | Lower complexity; focused on specific cell or tissue-level mechanisms. | Higher complexity; integrates whole-organism responses and multiple biological systems. |
| Data Type | Molecular, cellular, and biochemical data. | Pharmacokinetics, toxicology, efficacy, and immunological data. |
| Advantages | Cost-effective, rapid, controlled variables, ethical simplicity. | Realistic biological response, systemic effects, clinical relevance. |
| Limitations | Lacks systemic interactions, may not predict in vivo outcomes. | Costly, time-consuming, ethical considerations, biological variability. |
Definition of In Vitro and In Vivo Methods
In vitro methods refer to experiments conducted outside a living organism, typically in controlled laboratory environments such as test tubes or petri dishes, enabling precise analysis of biological processes at the cellular or molecular level. In vivo methods involve testing within living organisms, allowing researchers to observe complex interactions and physiological responses in a natural biological context. Both methods play crucial roles in medical device testing, with in vitro offering initial controlled data and in vivo providing comprehensive insights into device safety and efficacy.
Key Differences Between In Vitro and In Vivo Testing
In vitro testing involves studying biological processes outside a living organism, typically in controlled laboratory environments such as petri dishes or test tubes, allowing precise observation of cellular or molecular interactions. In vivo testing evaluates the effects of medical devices or treatments within living organisms, providing comprehensive insights into complex biological systems and potential systemic responses. Key differences include the environment of testing, with in vitro offering controlled but simplified conditions, while in vivo presents physiological relevance and complexity critical for assessing biocompatibility and device performance.
Advantages of In Vitro Testing in Medical Devices
In vitro testing allows for precise control of experimental conditions, enabling detailed analysis of medical device interactions with biological tissues without ethical concerns related to human or animal subjects. This method accelerates the development process by providing rapid and reproducible results, reducing costs compared to in vivo testing. Moreover, in vitro assays facilitate high-throughput screening and mechanistic understanding, improving device safety and efficacy before clinical trials.
Benefits of In Vivo Testing for Medical Device Evaluation
In vivo testing provides critical insights into the biocompatibility, safety, and functional performance of medical devices within a living organism, reflecting complex biological interactions unattainable in vitro. It enables real-time assessment of device integration, immune response, and systemic effects over extended periods, essential for regulatory approval and clinical success. Data derived from in vivo studies substantially enhance the predictive validity and reliability of medical device evaluations, guiding design optimization and risk mitigation.
Regulatory Requirements for In Vitro vs In Vivo Data
Regulatory requirements for in vitro data typically emphasize analytical validity, reproducibility, and standardized assay protocols to ensure accurate biomarker or device performance assessment under controlled laboratory conditions. In vivo data must demonstrate safety and efficacy within a living organism, requiring comprehensive preclinical studies and clinical trials that comply with Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) guidelines. Regulatory bodies such as the FDA and EMA enforce distinct submission criteria for in vitro diagnostics (IVDs) and in vivo medical devices, mandating rigorous evidence of clinical benefit and risk management tailored to each modality.
Challenges of Translating In Vitro Results to In Vivo Outcomes
In vitro studies provide controlled environments to assess the safety and efficacy of medical devices, but translating these results to in vivo outcomes presents significant challenges due to the complex biological interactions in living organisms. Factors such as immune response, tissue heterogeneity, and metabolic processes can alter device performance and biocompatibility, often leading to discrepancies between in vitro predictions and actual in vivo behavior. Understanding these challenges is critical for improving preclinical testing models and enhancing the reliability of medical device development.
Case Studies: In Vitro and In Vivo Approaches in Device Development
Case studies in medical device development illustrate the complementary roles of in vitro and in vivo approaches, where in vitro testing allows for precise control and rapid screening of biocompatibility and cytotoxicity, while in vivo studies provide critical insights into device performance, integration, and safety within complex biological systems. Devices such as implantable cardiac pacemakers undergo extensive in vitro electrical and mechanical testing to optimize design parameters before proceeding to in vivo animal models that evaluate physiological responses and long-term biocompatibility. The integration of data from both methodologies is essential for regulatory approval processes and ensures the efficacy and safety of medical devices in clinical applications.
Risk Assessment: When to Choose In Vitro or In Vivo Testing
Risk assessment in medical device development dictates the choice between in vitro and in vivo testing based on the complexity and biological relevance of the evaluation needed. In vitro testing offers controlled environments for initial toxicity and biocompatibility screening, reducing ethical concerns and costs. In vivo testing is essential when systemic interactions, long-term effects, or complex biological processes must be assessed to ensure safety and efficacy before human trials.
Ethical Considerations in In Vivo Medical Device Testing
In vivo medical device testing involves direct experimentation on living organisms, raising significant ethical considerations such as animal welfare, informed consent, and minimizing harm. Regulatory frameworks like the Declaration of Helsinki and Institutional Review Boards (IRBs) enforce strict guidelines to ensure patient safety and ethical compliance. Alternatives like in vitro testing and computer simulations are increasingly prioritized to reduce reliance on in vivo trials while maintaining rigorous safety assessments.
Future Trends: Innovations in In Vitro and In Vivo Evaluation Methods
Emerging innovations in in vitro and in vivo evaluation methods are transforming medical device testing by integrating advanced technologies such as organ-on-a-chip models and AI-driven data analysis to enhance predictive accuracy and reduce reliance on animal testing. Future trends emphasize the development of microphysiological systems that closely mimic human biological responses, enabling faster and more ethical device validation. These advancements aim to improve safety assessments and regulatory compliance while accelerating medical device innovation cycles.
In vitro vs In vivo Infographic
 productdif.com
productdif.com