Combination products integrate medical devices with drugs or biologics to enhance treatment efficacy and streamline therapy delivery. Standalone products function independently, offering targeted solutions without the need for other therapeutic components. Understanding the regulatory pathways and clinical benefits of each is crucial for optimizing patient outcomes and compliance.
Table of Comparison
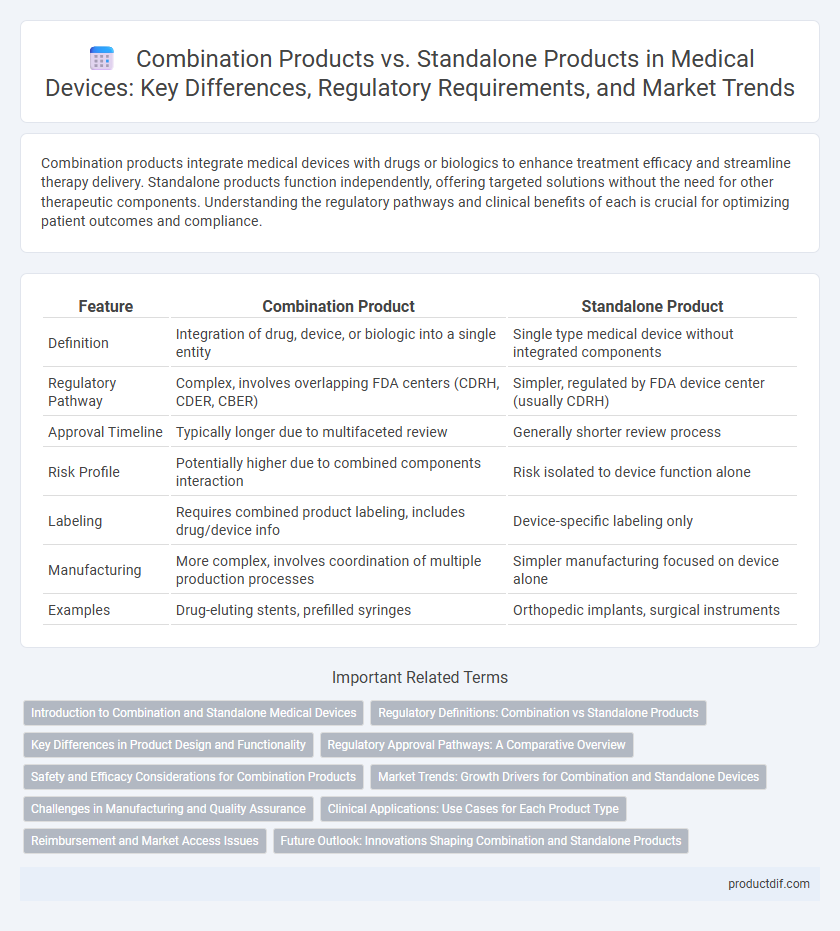
| Feature | Combination Product | Standalone Product |
|---|---|---|
| Definition | Integration of drug, device, or biologic into a single entity | Single type medical device without integrated components |
| Regulatory Pathway | Complex, involves overlapping FDA centers (CDRH, CDER, CBER) | Simpler, regulated by FDA device center (usually CDRH) |
| Approval Timeline | Typically longer due to multifaceted review | Generally shorter review process |
| Risk Profile | Potentially higher due to combined components interaction | Risk isolated to device function alone |
| Labeling | Requires combined product labeling, includes drug/device info | Device-specific labeling only |
| Manufacturing | More complex, involves coordination of multiple production processes | Simpler manufacturing focused on device alone |
| Examples | Drug-eluting stents, prefilled syringes | Orthopedic implants, surgical instruments |
Introduction to Combination and Standalone Medical Devices
Combination medical devices integrate drug, device, or biological elements, enhancing therapeutic effectiveness by providing targeted delivery or multifunctional treatment capabilities. Standalone medical devices operate independently without incorporating drugs or biological components, focusing solely on mechanical or technological functions for diagnosis, monitoring, or therapy. Regulatory pathways for combination products often demand compliance with both drug and device standards, creating distinct approval processes compared to standalone devices.
Regulatory Definitions: Combination vs Standalone Products
Combination products integrate drug, device, and/or biological components, requiring regulatory oversight from multiple authorities such as the FDA's Office of Combination Products. Standalone products consist of a single modality, either solely a medical device, drug, or biologic, and follow the distinct regulatory pathway specific to that category. Regulatory definitions emphasize the primary mode of action to determine the lead center and applicable compliance standards for combination versus standalone products.
Key Differences in Product Design and Functionality
Combination products integrate drug, device, and/or biological components, requiring complex design to ensure compatibility and coordinated functionality, whereas standalone products are singular in nature with isolated functionality. The design of combination products necessitates rigorous testing for synergistic effects and regulatory compliance across multiple product categories. Functionality in combination products often aims to enhance therapeutic outcomes through integrated mechanisms, while standalone devices focus solely on specific operational tasks or treatments.
Regulatory Approval Pathways: A Comparative Overview
Combination products, integrating medical devices with drugs or biologics, require coordinated regulatory approval pathways involving multiple agencies such as the FDA's Office of Combination Products to ensure safety and efficacy. Standalone medical devices follow a more straightforward approval process, typically through 510(k) clearance, PMA, or de novo classification depending on risk level. The complexity of combination product approvals often results in longer review timelines and necessitates comprehensive documentation addressing both device and drug or biologic components.
Safety and Efficacy Considerations for Combination Products
Combination products integrate drug, device, and/or biological components, requiring rigorous evaluation of both individual elements and their interactions to ensure safety and efficacy. The assessment process includes detailed risk analysis, compatibility testing, and clinical trials to address potential adverse effects arising from component integration. Regulatory frameworks, such as those established by the FDA, mandate comprehensive data demonstrating that the combined product performs safely and effectively as a unified system rather than as separate entities.
Market Trends: Growth Drivers for Combination and Standalone Devices
The market for combination products, integrating drugs, devices, and biologics, is rapidly expanding due to enhanced therapeutic efficacy and regulatory support from agencies like the FDA. Growth drivers for standalone medical devices include technological advancements in diagnostic accuracy, increasing prevalence of chronic diseases, and rising demand for minimally invasive procedures. Both segments benefit from growing healthcare expenditure globally and the shift towards personalized medicine, which fuels innovation and adoption in the medical technology landscape.
Challenges in Manufacturing and Quality Assurance
Manufacturing combination products involves integrating drug, device, and biologic components, which complicates regulatory compliance and quality assurance due to differing standards and stringent validation requirements for each component. Ensuring consistent performance and compatibility between the constituent elements demands robust risk management and specialized production processes to prevent cross-contamination and maintain product integrity. Quality assurance systems must adapt to oversee complex supply chains, enforce rigorous testing protocols, and address challenges in traceability and stability that are less prevalent with standalone medical devices.
Clinical Applications: Use Cases for Each Product Type
Combination products integrate drug, device, or biologic components to enhance treatment efficacy in complex clinical scenarios such as drug-eluting stents for cardiovascular disease and prefilled syringes for targeted drug delivery. Standalone medical devices, including diagnostic imaging systems and orthopedic implants, offer specialized functionality without pharmacological components, excelling in applications like fracture stabilization and non-invasive diagnostics. Selecting between combination and standalone products depends on clinical objectives, with combination products enabling multimodal therapy and standalone devices providing focused mechanical or diagnostic interventions.
Reimbursement and Market Access Issues
Combination products often face complex reimbursement challenges due to their dual nature, involving both device and pharmaceutical components that require distinct coding and coverage policies. Standalone products typically benefit from more straightforward market access pathways with established reimbursement frameworks, accelerating time-to-market and reducing payer uncertainty. Navigating payer requirements and demonstrating combined product value through clinical and economic evidence is crucial for securing favorable reimbursement outcomes.
Future Outlook: Innovations Shaping Combination and Standalone Products
Emerging innovations in medical technology are driving the evolution of combination products by integrating advanced drug delivery systems with wearable sensors, enhancing personalized treatment efficacy. Standalone medical devices are increasingly incorporating AI and machine learning algorithms to improve diagnostic accuracy and patient monitoring. The future landscape anticipates a convergence where both product types leverage digital health advancements to optimize therapeutic outcomes and streamline regulatory pathways.
Combination product vs Standalone product Infographic
 productdif.com
productdif.com