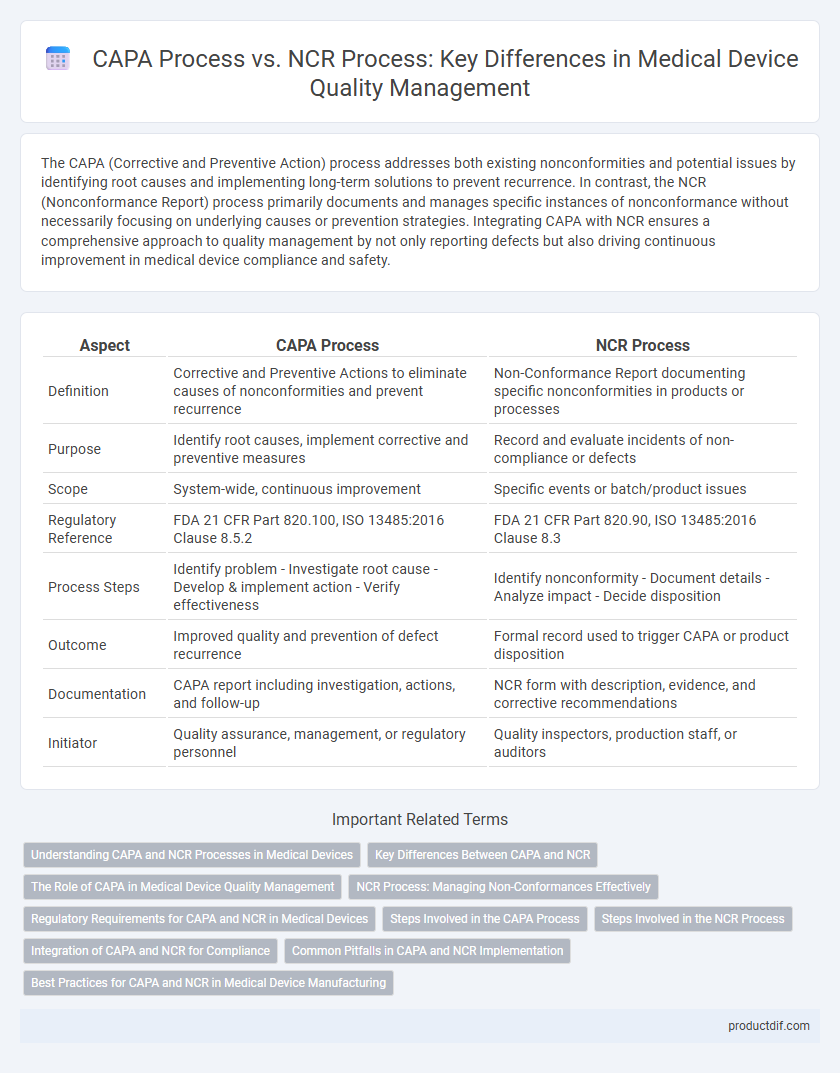
The CAPA (Corrective and Preventive Action) process addresses both existing nonconformities and potential issues by identifying root causes and implementing long-term solutions to prevent recurrence. In contrast, the NCR (Nonconformance Report) process primarily documents and manages specific instances of nonconformance without necessarily focusing on underlying causes or prevention strategies. Integrating CAPA with NCR ensures a comprehensive approach to quality management by not only reporting defects but also driving continuous improvement in medical device compliance and safety.
Table of Comparison
| Aspect | CAPA Process | NCR Process |
|---|---|---|
| Definition | Corrective and Preventive Actions to eliminate causes of nonconformities and prevent recurrence | Non-Conformance Report documenting specific nonconformities in products or processes |
| Purpose | Identify root causes, implement corrective and preventive measures | Record and evaluate incidents of non-compliance or defects |
| Scope | System-wide, continuous improvement | Specific events or batch/product issues |
| Regulatory Reference | FDA 21 CFR Part 820.100, ISO 13485:2016 Clause 8.5.2 | FDA 21 CFR Part 820.90, ISO 13485:2016 Clause 8.3 |
| Process Steps | Identify problem - Investigate root cause - Develop & implement action - Verify effectiveness | Identify nonconformity - Document details - Analyze impact - Decide disposition |
| Outcome | Improved quality and prevention of defect recurrence | Formal record used to trigger CAPA or product disposition |
| Documentation | CAPA report including investigation, actions, and follow-up | NCR form with description, evidence, and corrective recommendations |
| Initiator | Quality assurance, management, or regulatory personnel | Quality inspectors, production staff, or auditors |
Understanding CAPA and NCR Processes in Medical Devices
The CAPA process in medical devices focuses on identifying, investigating, and correcting non-conformities or quality issues to prevent recurrence, ensuring ongoing compliance with regulatory standards such as FDA 21 CFR Part 820. The NCR process documents specific instances of non-conformance, serving as a trigger for CAPA actions by detailing deviations from established specifications or procedures. Understanding the integration of CAPA and NCR processes is critical for effective quality management systems, risk mitigation, and maintaining product safety in medical device manufacturing.
Key Differences Between CAPA and NCR
The CAPA (Corrective and Preventive Action) process focuses on identifying, investigating, and eliminating root causes of actual and potential nonconformities to prevent recurrence, while the NCR (Non-Conformance Report) process documents and manages specific instances of nonconformity detected in medical devices. CAPA encompasses a broader scope involving risk assessment, root cause analysis, and systemic improvements, whereas NCR is primarily a reporting tool that triggers further investigation or CAPA when necessary. The effectiveness of CAPA lies in its preventive approach, while NCR serves as an essential initial step for quality control and regulatory compliance in medical device manufacturing.
The Role of CAPA in Medical Device Quality Management
The Corrective and Preventive Action (CAPA) process plays a critical role in medical device quality management by systematically identifying, investigating, and addressing root causes of non-conformities to prevent recurrence. While the Non-Conformance Report (NCR) process documents specific deviations from quality standards, CAPA ensures these issues are resolved through long-term corrective measures and risk mitigation strategies. Implementing an effective CAPA process enhances regulatory compliance with FDA and ISO 13485 requirements, ultimately improving product safety and reliability in the medical device industry.
NCR Process: Managing Non-Conformances Effectively
The Non-Conformance Report (NCR) process is critical for identifying, documenting, and addressing deviations from medical device quality standards, ensuring compliance with regulatory requirements such as ISO 13485 and FDA 21 CFR Part 820. Effective NCR management involves root cause analysis, containment actions, and corrective measures to prevent product quality issues and maintain patient safety. Integrating NCR processes with quality management systems enhances traceability, promotes continuous improvement, and reduces the risk of device recalls.
Regulatory Requirements for CAPA and NCR in Medical Devices
Regulatory requirements for CAPA (Corrective and Preventive Action) in medical devices mandate prompt identification, investigation, and remediation of nonconformities to ensure device safety and compliance with standards like ISO 13485 and FDA 21 CFR Part 820. The NCR (Non-Conformance Report) process documents deviations from specifications but serves primarily as an initial record rather than a comprehensive corrective framework. Effective CAPA integrates NCR data to address root causes, prevent recurrence, and maintain regulatory compliance throughout the device lifecycle.
Steps Involved in the CAPA Process
The CAPA process in medical device management involves identifying and analyzing nonconformities, investigating root causes, and implementing corrective and preventive actions to prevent recurrence. Key steps include problem identification, risk assessment, action plan development, implementation, and effectiveness verification. This structured approach ensures compliance with regulatory standards such as FDA 21 CFR Part 820 and ISO 13485, enhancing device safety and quality.
Steps Involved in the NCR Process
The Non-Conformance Report (NCR) process in medical device manufacturing involves identifying, documenting, and evaluating deviations from specified standards or procedures, followed by containment actions to prevent further issues. Root cause analysis is performed to determine underlying factors, enabling corrective measures and verification of effectiveness to prevent recurrence. This structured approach ensures compliance with regulatory requirements and maintains product quality within the Corrective and Preventive Action (CAPA) framework.
Integration of CAPA and NCR for Compliance
Integrating Corrective and Preventive Action (CAPA) with Non-Conformance Report (NCR) processes streamlines quality management in medical device manufacturing, ensuring regulatory compliance with FDA and ISO 13485 standards. Effective CAPA-NCR integration accelerates root cause analysis and resolution, reducing recurrence of non-conformities and enhancing product safety and reliability. Automated tracking systems enable seamless data flow between CAPA and NCR records, facilitating audit readiness and continuous improvement initiatives.
Common Pitfalls in CAPA and NCR Implementation
Common pitfalls in CAPA (Corrective and Preventive Action) and NCR (Non-Conformance Report) implementation include inadequate root cause analysis, leading to recurring quality issues and unresolved compliance risks. Failure to establish clear documentation and traceability often results in incomplete records, which complicates audits and regulatory inspections within medical device manufacturing. Insufficient training of personnel on CAPA and NCR procedures compromises the effectiveness of corrective actions and delays product release timelines, ultimately affecting patient safety and regulatory adherence.
Best Practices for CAPA and NCR in Medical Device Manufacturing
Best practices for the CAPA (Corrective and Preventive Action) process in medical device manufacturing emphasize thorough root cause analysis, timely implementation of corrective measures, and continuous monitoring to prevent recurrence of non-conformities. The NCR (Non-Conformance Report) process requires meticulous documentation, rapid identification of deviations from product specifications, and prompt segregation of affected batches to maintain product integrity. Integrating both CAPA and NCR processes within a robust quality management system ensures regulatory compliance and enhances overall product safety and efficacy.
CAPA process vs NCR process Infographic
 productdif.com
productdif.com