Class I medical devices are generally low-risk instruments subject to minimal regulatory controls, ensuring safety and effectiveness through basic quality management systems. Class II devices carry moderate risk and require more stringent regulatory oversight, including premarket notification and compliance with specific performance standards. Understanding the classification helps manufacturers implement appropriate risk management and regulatory strategies to meet FDA requirements.
Table of Comparison
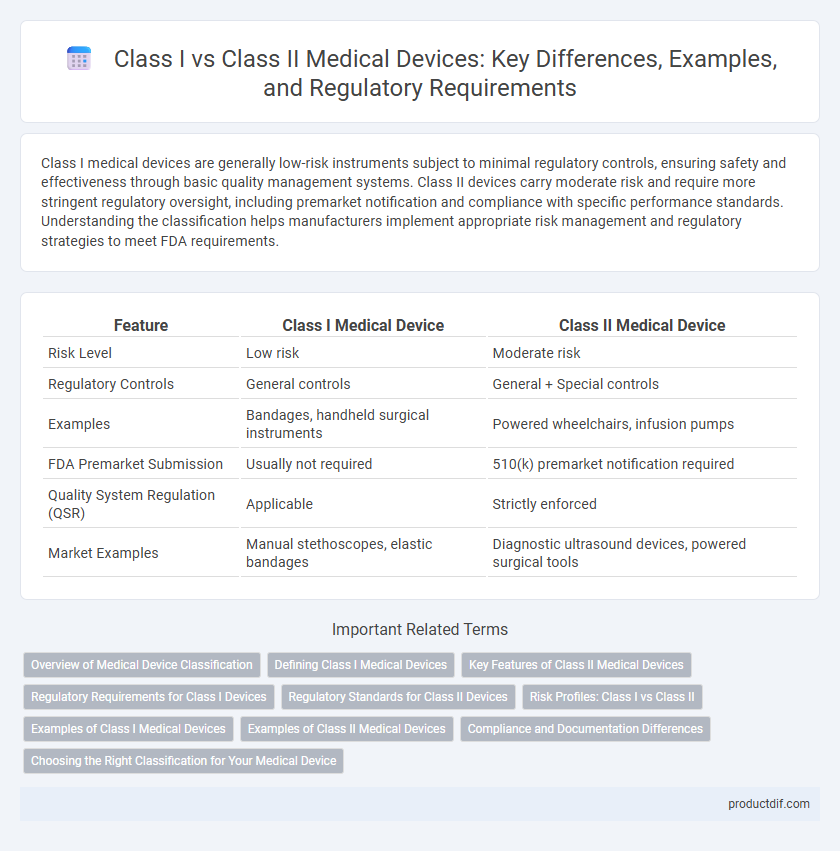
| Feature | Class I Medical Device | Class II Medical Device |
|---|---|---|
| Risk Level | Low risk | Moderate risk |
| Regulatory Controls | General controls | General + Special controls |
| Examples | Bandages, handheld surgical instruments | Powered wheelchairs, infusion pumps |
| FDA Premarket Submission | Usually not required | 510(k) premarket notification required |
| Quality System Regulation (QSR) | Applicable | Strictly enforced |
| Market Examples | Manual stethoscopes, elastic bandages | Diagnostic ultrasound devices, powered surgical tools |
Overview of Medical Device Classification
Medical device classification categorizes devices based on risk levels to ensure appropriate regulatory control. Class I devices pose the lowest risk and typically require general controls, while Class II devices present moderate risk and usually demand special controls including performance standards and postmarket surveillance. Understanding the distinction between Class I and Class II is critical for manufacturers to comply with FDA regulations and streamline the approval process.
Defining Class I Medical Devices
Class I medical devices are low-risk products subject to the least regulatory control by the FDA, typically including items like bandages, examination gloves, and hand-held surgical instruments. These devices usually do not require premarket approval but must comply with general controls such as proper labeling, manufacturing practices, and registration. Understanding the classification helps manufacturers navigate regulatory requirements and ensures patient safety.
Key Features of Class II Medical Devices
Class II medical devices are characterized by moderate to high risk and require greater regulatory controls than Class I devices, including premarket notification (510(k)) to demonstrate safety and effectiveness. These devices often involve complex technology, such as infusion pumps and surgical drapes, requiring specific performance standards and post-market surveillance. Enhanced labeling, quality system regulations, and adherence to FDA guidance documents are critical for Class II medical device compliance.
Regulatory Requirements for Class I Devices
Class I medical devices, classified by the FDA as low-risk products, are subject to general controls such as proper labeling, manufacturing practices under 21 CFR Part 820, and registration and listing requirements. Unlike Class II devices, Class I devices typically do not require premarket notification (510(k)) unless specifically mandated, streamlining their regulatory pathway. Compliance with the Quality System Regulation (QSR) and adherence to applicable standards ensure the safety and effectiveness of Class I medical devices in the marketplace.
Regulatory Standards for Class II Devices
Class II medical devices require compliance with more stringent regulatory standards compared to Class I, including premarket notification through 510(k) submissions to the FDA. These devices must adhere to specific performance standards, postmarket surveillance, and quality system regulations to ensure safety and effectiveness. Regulatory oversight for Class II devices involves rigorous testing, labeling requirements, and adherence to ISO 13485 quality management standards.
Risk Profiles: Class I vs Class II
Class I medical devices generally present low risk and are subject to the least regulatory controls, typically including basic labeling and manufacturing standards. Class II devices carry moderate risk and require more stringent regulatory controls, such as premarket notification (510(k)) and special performance standards to ensure safety and effectiveness. Understanding the distinct risk profiles between Class I and Class II is essential for manufacturers to comply with FDA regulatory requirements and mitigate potential patient harm.
Examples of Class I Medical Devices
Class I medical devices include everyday items such as bandages, examination gloves, and hand-held surgical instruments, characterized by low risk and general controls. These devices do not require premarket approval but must comply with FDA regulations to ensure safety and effectiveness. Examples highlight their frequent use in basic healthcare settings and support routine medical procedures.
Examples of Class II Medical Devices
Class II medical devices include powered wheelchairs, infusion pumps, and surgical drapes, which require greater regulatory control than Class I due to moderate risk levels. These devices often involve complex mechanical or electronic components that necessitate performance standards and postmarket surveillance. The U.S. Food and Drug Administration (FDA) mandates premarket notification 510(k) submissions for Class II devices to ensure safety and effectiveness before market approval.
Compliance and Documentation Differences
Class I medical devices require general controls including basic labeling, manufacturing practices, and registration, with minimal documentation compared to Class II devices. Class II devices demand compliance with special controls such as performance standards, postmarket surveillance, and more comprehensive premarket notification (510(k)), resulting in detailed technical documentation. These stringent requirements ensure higher safety and effectiveness standards for Class II devices, reflecting their increased risk level.
Choosing the Right Classification for Your Medical Device
Choosing the right classification for your medical device is crucial to ensure compliance with regulatory requirements and market access. Class I devices typically pose low risk and have general controls, while Class II devices carry moderate risk and require special controls such as performance standards and post-market surveillance. Understanding the device's intended use, risk profile, and regulatory guidelines from authorities like the FDA or EU MDR helps determine the appropriate classification and streamline the approval process.
Class I vs Class II Infographic
 productdif.com
productdif.com