Single-use medical devices offer enhanced infection control by minimizing cross-contamination risks compared to multi-use devices, which require thorough sterilization between uses to ensure patient safety. Multi-use devices provide cost-effectiveness and sustainability benefits by reducing waste, but they demand stringent maintenance protocols to maintain functionality and hygiene standards. Selecting between single-use and multi-use devices depends on clinical application, budget considerations, and regulatory requirements.
Table of Comparison
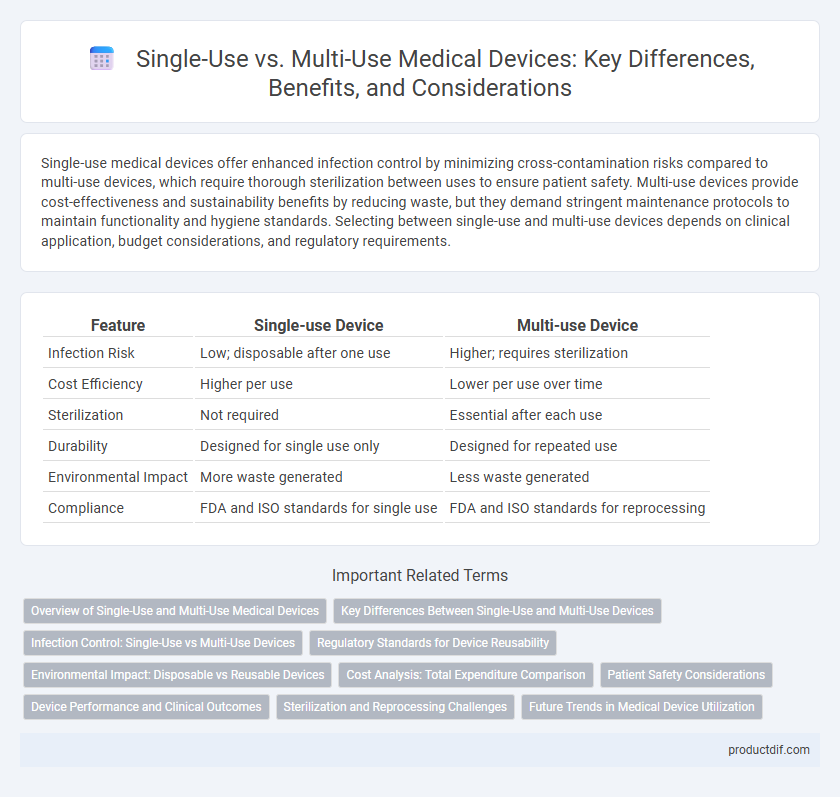
| Feature | Single-use Device | Multi-use Device |
|---|---|---|
| Infection Risk | Low; disposable after one use | Higher; requires sterilization |
| Cost Efficiency | Higher per use | Lower per use over time |
| Sterilization | Not required | Essential after each use |
| Durability | Designed for single use only | Designed for repeated use |
| Environmental Impact | More waste generated | Less waste generated |
| Compliance | FDA and ISO standards for single use | FDA and ISO standards for reprocessing |
Overview of Single-Use and Multi-Use Medical Devices
Single-use medical devices are designed for one-time use and disposal to prevent cross-contamination and infection, ensuring patient safety through sterile functionality. Multi-use devices undergo rigorous sterilization processes between uses, offering cost-effectiveness and sustainability in clinical settings while requiring strict adherence to cleaning protocols. Regulatory guidelines from agencies like the FDA emphasize validation of reprocessing methods for multi-use devices to maintain safety and efficacy standards.
Key Differences Between Single-Use and Multi-Use Devices
Single-use medical devices are designed for one-time use and are typically disposable to prevent cross-contamination and infection risks, whereas multi-use devices are intended for repeated use and require thorough cleaning, sterilization, and maintenance between uses. Single-use devices offer convenience and reduce the risk of hospital-acquired infections, while multi-use devices provide cost-effectiveness and sustainability through durability and reusability. Regulatory standards emphasize stringent validation processes for sterilization methods on multi-use devices, making compliance critical for patient safety and device longevity.
Infection Control: Single-Use vs Multi-Use Devices
Single-use medical devices significantly reduce infection risks by eliminating the need for sterilization between patients, thereby preventing cross-contamination. Multi-use devices require rigorous cleaning and sterilization protocols to maintain infection control, but errors in these processes can lead to pathogen transmission. Studies indicate that single-use devices are associated with lower rates of healthcare-associated infections compared to multi-use counterparts, particularly in high-risk procedures.
Regulatory Standards for Device Reusability
Regulatory standards for single-use and multi-use medical devices differ significantly, with single-use devices typically requiring validation to ensure safe disposal after one use to prevent contamination and infection. Multi-use devices must comply with stringent cleaning, disinfection, and sterilization protocols established by agencies like the FDA and ISO 17664 to guarantee patient safety across multiple uses. Compliance with these standards involves rigorous documentation, risk assessments, and validation of reprocessing methods to meet regulatory requirements for device reusability.
Environmental Impact: Disposable vs Reusable Devices
Single-use medical devices generate significant medical waste, contributing to environmental pollution and increased landfill burden due to their disposable nature. In contrast, multi-use devices reduce waste output through sterilization and reprocessing, lowering overall environmental footprint by minimizing resource consumption and hazardous waste. Lifecycle assessments of reusable devices reveal substantial reductions in carbon emissions and raw material use compared to single-use counterparts.
Cost Analysis: Total Expenditure Comparison
Single-use medical devices typically incur higher per-unit costs due to their disposable nature but eliminate expenses related to sterilization and reprocessing. Multi-use devices require upfront investment and ongoing costs for sterilization, maintenance, and potential repair, affecting the total expenditure over time. A comprehensive cost analysis must consider acquisition, reprocessing, and infection risk-related expenses to determine the most economical choice in clinical settings.
Patient Safety Considerations
Single-use devices significantly reduce the risk of cross-contamination and infection, enhancing patient safety by eliminating the need for sterilization between uses. Multi-use devices require stringent sterilization protocols to prevent pathogen transmission, which can be compromised by human error or equipment failure. Choosing single-use devices is often preferred in high-risk clinical environments to ensure consistent aseptic conditions and minimize healthcare-associated infections (HAIs).
Device Performance and Clinical Outcomes
Single-use medical devices eliminate cross-contamination risks, ensuring consistent device performance and reducing infection rates in clinical outcomes. Multi-use devices may experience performance variability due to repeated sterilization, potentially compromising reliability and increasing the risk of device-related complications. Studies indicate single-use devices often lead to improved patient safety and lower healthcare-associated infection rates compared to multi-use alternatives.
Sterilization and Reprocessing Challenges
Single-use medical devices eliminate the need for sterilization, reducing patient infection risks and ensuring device integrity, as they are designed for single patient use only. Multi-use devices require rigorous and validated reprocessing protocols, including cleaning, sterilization, and inspection, to prevent cross-contamination and maintain functionality. Challenges in sterilization arise from device complexity, material compatibility, and detection of residual contaminants, which can compromise patient safety and regulatory compliance.
Future Trends in Medical Device Utilization
Future trends in medical device utilization emphasize the increasing adoption of single-use devices due to enhanced infection control and regulatory standards promoting patient safety. Advances in biodegradable materials and cost-effective manufacturing technologies are expected to reduce environmental impact associated with disposable devices. Multi-use devices will continue evolving with improvements in sterilization methods and smart tracking systems to extend device lifespan while maintaining compliance with stringent healthcare protocols.
Single-use device vs Multi-use device Infographic
 productdif.com
productdif.com