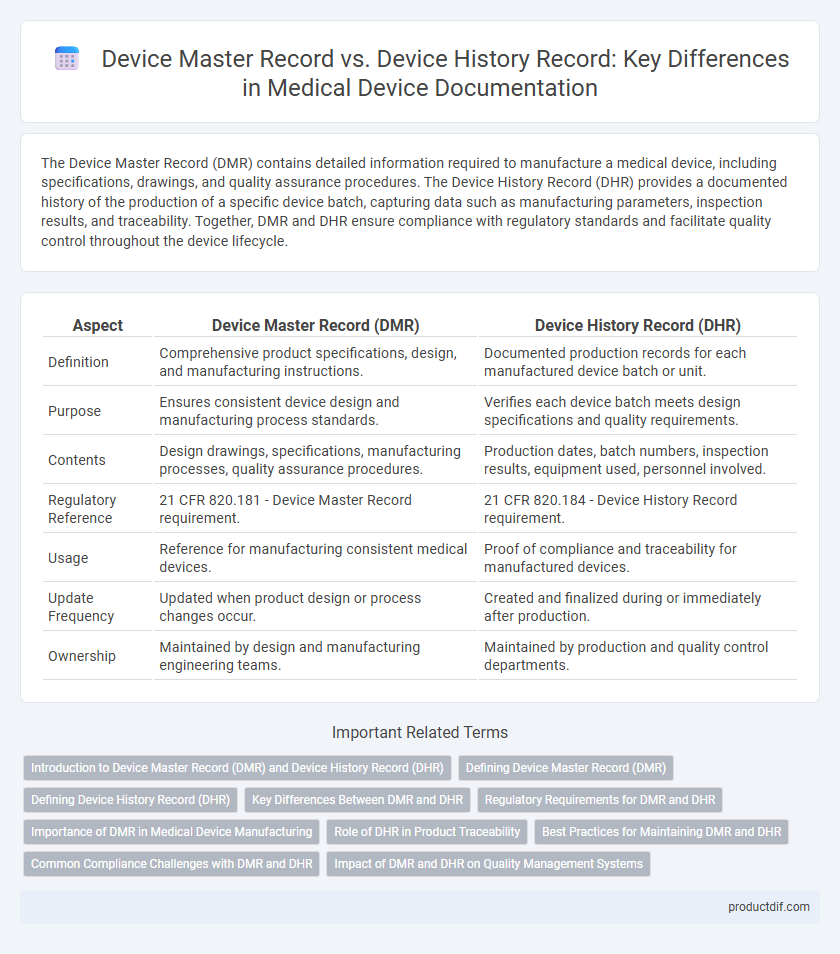
The Device Master Record (DMR) contains detailed information required to manufacture a medical device, including specifications, drawings, and quality assurance procedures. The Device History Record (DHR) provides a documented history of the production of a specific device batch, capturing data such as manufacturing parameters, inspection results, and traceability. Together, DMR and DHR ensure compliance with regulatory standards and facilitate quality control throughout the device lifecycle.
Table of Comparison
| Aspect | Device Master Record (DMR) | Device History Record (DHR) |
|---|---|---|
| Definition | Comprehensive product specifications, design, and manufacturing instructions. | Documented production records for each manufactured device batch or unit. |
| Purpose | Ensures consistent device design and manufacturing process standards. | Verifies each device batch meets design specifications and quality requirements. |
| Contents | Design drawings, specifications, manufacturing processes, quality assurance procedures. | Production dates, batch numbers, inspection results, equipment used, personnel involved. |
| Regulatory Reference | 21 CFR 820.181 - Device Master Record requirement. | 21 CFR 820.184 - Device History Record requirement. |
| Usage | Reference for manufacturing consistent medical devices. | Proof of compliance and traceability for manufactured devices. |
| Update Frequency | Updated when product design or process changes occur. | Created and finalized during or immediately after production. |
| Ownership | Maintained by design and manufacturing engineering teams. | Maintained by production and quality control departments. |
Introduction to Device Master Record (DMR) and Device History Record (DHR)
The Device Master Record (DMR) contains the comprehensive specifications, manufacturing processes, and quality assurance procedures necessary for producing a medical device, serving as the authoritative reference throughout production. The Device History Record (DHR) documents the actual manufacturing history of each specific device batch, capturing critical data such as production dates, personnel involved, and quality control results to ensure traceability and regulatory compliance. Both DMR and DHR are essential for meeting FDA requirements under 21 CFR Part 820, facilitating consistent device quality and enabling effective post-market surveillance.
Defining Device Master Record (DMR)
The Device Master Record (DMR) is a comprehensive compilation of all the detailed specifications, drawings, formulations, and instructions necessary to manufacture a medical device, serving as the authoritative reference for production. It includes the product specifications, production process specifications, quality assurance procedures, and packaging and labeling specifications. The DMR ensures consistency in device manufacturing and regulatory compliance by providing a complete blueprint for producing medical devices that meet safety and performance standards.
Defining Device History Record (DHR)
The Device History Record (DHR) is a comprehensive compilation of production data capturing the manufacturing details for each unit of a medical device, ensuring traceability and compliance with regulatory standards such as FDA 21 CFR Part 820. Unlike the Device Master Record (DMR), which contains the specifications and procedures for device production, the DHR documents the unique production run, including batch records, inspection results, and labeling materials used. Maintaining an accurate DHR is critical for quality control, post-market surveillance, and facilitating corrective actions in medical device manufacturing.
Key Differences Between DMR and DHR
The Device Master Record (DMR) contains comprehensive documentation of a medical device's design, specifications, and manufacturing processes, serving as the blueprint for production. In contrast, the Device History Record (DHR) provides a detailed record of the manufacturing history for each batch or unit, including inspection results and traceability data. Key differences between DMR and DHR include DMR being a static compilation that guides manufacturing, while DHR is a dynamic report capturing actual production activities and quality control outcomes.
Regulatory Requirements for DMR and DHR
The Device Master Record (DMR) contains comprehensive documentation required by FDA 21 CFR 820.181 for manufacturing specifications, procedures, and quality assurance of medical devices. The Device History Record (DHR) must comply with 21 CFR 820.184 by including actual production data and records that verify each unit was made according to the DMR. Regulatory requirements mandate rigorous control and traceability to ensure device safety, effectiveness, and conformity with Good Manufacturing Practices (GMP).
Importance of DMR in Medical Device Manufacturing
The Device Master Record (DMR) contains comprehensive documentation including product specifications, manufacturing processes, quality assurance procedures, and packaging details critical for consistent medical device production. Maintaining an accurate DMR ensures regulatory compliance with FDA and ISO standards, facilitating traceability and quality control throughout the product lifecycle. The DMR serves as the authoritative source for manufacturing information, directly impacting the safety, reliability, and effectiveness of medical devices in healthcare settings.
Role of DHR in Product Traceability
The Device History Record (DHR) plays a crucial role in product traceability by providing a comprehensive and chronological documentation of the manufacturing history for each individual medical device. Unlike the Device Master Record (DMR), which contains standardized manufacturing specifications and procedures, the DHR captures batch-specific data, including production dates, quality control results, and operator details. This traceability ensures regulatory compliance and facilitates timely identification and resolution of quality issues or recalls in the medical device industry.
Best Practices for Maintaining DMR and DHR
Maintaining a Device Master Record (DMR) and Device History Record (DHR) requires strict adherence to regulatory standards such as FDA 21 CFR Part 820 to ensure traceability and product compliance. Best practices include implementing robust version control for the DMR to document detailed specifications, drawings, and quality assurance procedures, while the DHR must accurately capture manufacturing processes, lot numbers, and inspection results for each device batch. Leveraging electronic quality management systems (eQMS) enhances the integrity and accessibility of both records, facilitating audits and minimizing risks related to device recalls or regulatory non-compliance.
Common Compliance Challenges with DMR and DHR
Device Master Record (DMR) and Device History Record (DHR) are critical components in medical device manufacturing that often face compliance challenges related to accuracy, completeness, and traceability. Common issues include maintaining up-to-date device specifications in the DMR, ensuring all manufacturing activities are accurately documented in the DHR, and aligning both records with regulatory requirements such as FDA 21 CFR Part 820. Failure to address discrepancies or incomplete data in DMR and DHR can result in audit findings, product recalls, and regulatory penalties.
Impact of DMR and DHR on Quality Management Systems
Device Master Record (DMR) provides the essential documentation for product specifications, manufacturing processes, and quality assurance, forming the foundation of a compliant Quality Management System (QMS). Device History Record (DHR) offers a comprehensive account of the production history for each device batch, ensuring traceability and verification of conformity to DMR requirements. Effective integration of DMR and DHR enhances regulatory compliance, reduces risk of errors, and supports continuous improvement within medical device Quality Management Systems.
Device Master Record vs Device History Record Infographic
 productdif.com
productdif.com