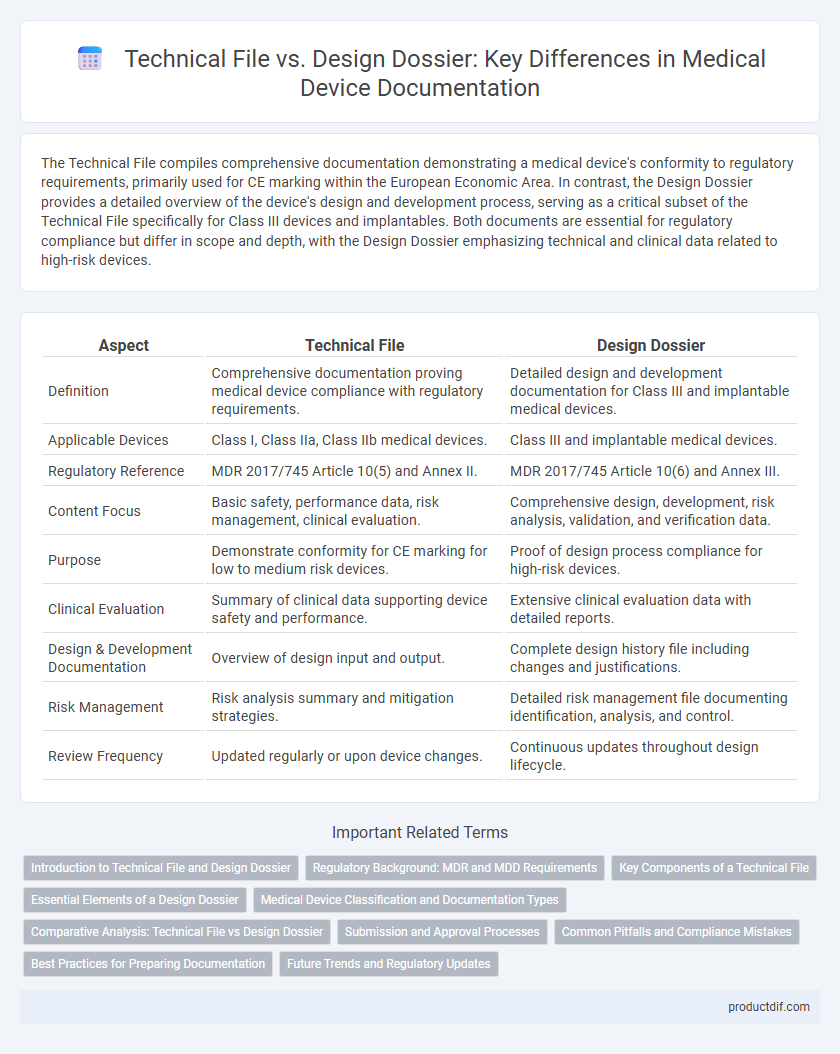
The Technical File compiles comprehensive documentation demonstrating a medical device's conformity to regulatory requirements, primarily used for CE marking within the European Economic Area. In contrast, the Design Dossier provides a detailed overview of the device's design and development process, serving as a critical subset of the Technical File specifically for Class III devices and implantables. Both documents are essential for regulatory compliance but differ in scope and depth, with the Design Dossier emphasizing technical and clinical data related to high-risk devices.
Table of Comparison
| Aspect | Technical File | Design Dossier |
|---|---|---|
| Definition | Comprehensive documentation proving medical device compliance with regulatory requirements. | Detailed design and development documentation for Class III and implantable medical devices. |
| Applicable Devices | Class I, Class IIa, Class IIb medical devices. | Class III and implantable medical devices. |
| Regulatory Reference | MDR 2017/745 Article 10(5) and Annex II. | MDR 2017/745 Article 10(6) and Annex III. |
| Content Focus | Basic safety, performance data, risk management, clinical evaluation. | Comprehensive design, development, risk analysis, validation, and verification data. |
| Purpose | Demonstrate conformity for CE marking for low to medium risk devices. | Proof of design process compliance for high-risk devices. |
| Clinical Evaluation | Summary of clinical data supporting device safety and performance. | Extensive clinical evaluation data with detailed reports. |
| Design & Development Documentation | Overview of design input and output. | Complete design history file including changes and justifications. |
| Risk Management | Risk analysis summary and mitigation strategies. | Detailed risk management file documenting identification, analysis, and control. |
| Review Frequency | Updated regularly or upon device changes. | Continuous updates throughout design lifecycle. |
Introduction to Technical File and Design Dossier
The Technical File is a comprehensive compilation of documents demonstrating a medical device's conformity with regulatory requirements, including design, manufacturing, and risk management data. The Design Dossier specifically contains detailed design and development information, serving as the primary evidence for product safety and performance. Both documents are essential for regulatory submissions and audits in the medical device industry.
Regulatory Background: MDR and MDD Requirements
The Medical Device Regulation (MDR) 2017/745 and the Medical Device Directive (MDD) 93/42/EEC establish distinct regulatory requirements for the Technical File and Design Dossier. A Technical File is mandated for Class I, IIa, and IIb devices under both MDR and MDD, containing comprehensive documentation proving conformity with essential safety and performance criteria. The Design Dossier is required specifically for Class III and implantable devices, providing detailed design, manufacturing processes, and risk management evidence to demonstrate compliance with stricter regulatory scrutiny.
Key Components of a Technical File
Key components of a Technical File include the device description, risk management files, clinical evaluation, and design verification and validation reports. This file contains essential documentation to demonstrate conformity with regulatory requirements, encompassing labeling, instructions for use, and post-market surveillance plans. Unlike a Design Dossier, the Technical File is more comprehensive, serving as evidence for both regulatory review and quality assurance throughout the product lifecycle.
Essential Elements of a Design Dossier
The Design Dossier compiles comprehensive documentation required to demonstrate conformity with regulatory standards, including detailed design specifications, risk assessments, and verification and validation reports. Essential elements feature the device description, manufacturing processes, clinical evaluation data, and labeling information, ensuring a thorough assessment of safety and performance. This dossier supports regulatory submissions, providing evidence for CE marking under the Medical Device Regulation (MDR) or other international requirements.
Medical Device Classification and Documentation Types
Medical device classification determines whether a Technical File or Design Dossier is required, with Class I devices typically necessitating a Technical File and Class IIa, IIb, and III devices requiring a Design Dossier. The Technical File contains documentation such as risk management, clinical evaluation, and manufacturing processes for lower-risk devices, while the Design Dossier includes detailed design specifications, verification, and validation data for higher-risk devices. Proper documentation based on classification ensures compliance with regulatory standards like the EU MDR and FDA guidelines.
Comparative Analysis: Technical File vs Design Dossier
The Technical File and Design Dossier serve as regulatory documentation for medical devices but differ in scope and application. A Technical File is typically required for Class I devices and includes information such as device description, risk assessment, and clinical data, emphasizing conformity with essential requirements. In contrast, the Design Dossier pertains to higher-risk devices (Class IIa, IIb, and III) and offers an extensive compilation of design and development details, verification and validation reports, and compliance evidence, supporting a more rigorous regulatory review process.
Submission and Approval Processes
The Technical File is primarily required for medical devices intended for the European market under the CE marking process, containing comprehensive documentation for conformity assessment and regulatory submission. In contrast, the Design Dossier is specifically mandated for Class III and implantable devices, detailing design, manufacturing, and testing data for notified body review during approval. Both submissions undergo rigorous evaluation to ensure compliance with the Medical Device Regulation (MDR) before receiving market authorization.
Common Pitfalls and Compliance Mistakes
Common pitfalls in managing Technical Files and Design Dossiers include incomplete documentation, inconsistent version control, and inadequate risk management records, which often lead to non-compliance with regulatory standards such as MDR 2017/745 or FDA 21 CFR Part 820. Failure to clearly distinguish between the Technical File--covering device specifications, manufacturing processes, and quality controls--and the Design Dossier--focused on detailed design and development data--can result in regulatory review delays or rejections. Ensuring comprehensive traceability, up-to-date evidence of conformity assessment, and thorough validation of design inputs and outputs is critical to avoid compliance mistakes during audits.
Best Practices for Preparing Documentation
Preparing a Technical File requires comprehensive documentation of the medical device's design, manufacturing processes, risk management, and compliance with relevant standards such as ISO 13485 and MDR 2017/745. A Design Dossier emphasizes detailed design validation, verification reports, and clinical evaluation, ensuring conformity to regulatory requirements and facilitating CE marking. Best practices include maintaining version control, ensuring traceability of changes, and conducting rigorous internal audits to verify completeness and accuracy of all documentation.
Future Trends and Regulatory Updates
Technical Files and Design Dossiers are evolving in response to stricter regulatory frameworks such as the EU MDR and IVDR, emphasizing increased transparency and comprehensive documentation. Future trends indicate a move towards digitalization and integration of real-world evidence to enhance post-market surveillance and risk management. Updated guidelines also stress the importance of lifecycle data management and cybersecurity considerations within both Technical Files and Design Dossiers to ensure ongoing compliance.
Technical File vs Design Dossier Infographic
 productdif.com
productdif.com