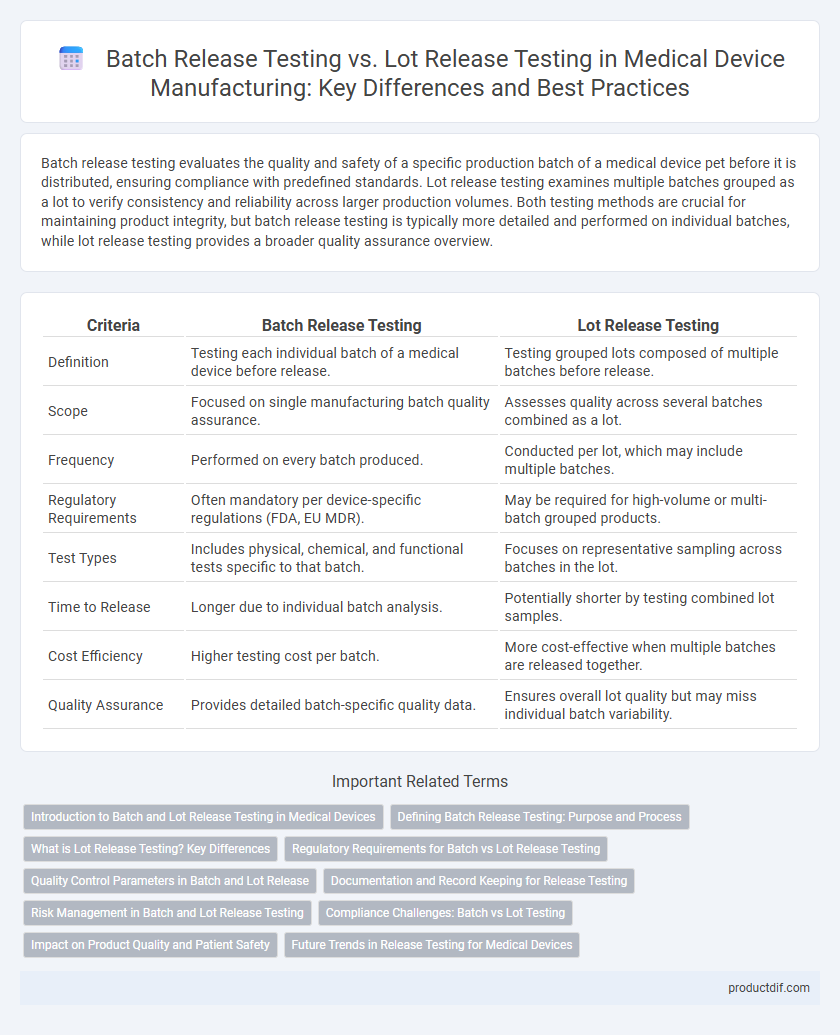
Batch release testing evaluates the quality and safety of a specific production batch of a medical device pet before it is distributed, ensuring compliance with predefined standards. Lot release testing examines multiple batches grouped as a lot to verify consistency and reliability across larger production volumes. Both testing methods are crucial for maintaining product integrity, but batch release testing is typically more detailed and performed on individual batches, while lot release testing provides a broader quality assurance overview.
Table of Comparison
| Criteria | Batch Release Testing | Lot Release Testing |
|---|---|---|
| Definition | Testing each individual batch of a medical device before release. | Testing grouped lots composed of multiple batches before release. |
| Scope | Focused on single manufacturing batch quality assurance. | Assesses quality across several batches combined as a lot. |
| Frequency | Performed on every batch produced. | Conducted per lot, which may include multiple batches. |
| Regulatory Requirements | Often mandatory per device-specific regulations (FDA, EU MDR). | May be required for high-volume or multi-batch grouped products. |
| Test Types | Includes physical, chemical, and functional tests specific to that batch. | Focuses on representative sampling across batches in the lot. |
| Time to Release | Longer due to individual batch analysis. | Potentially shorter by testing combined lot samples. |
| Cost Efficiency | Higher testing cost per batch. | More cost-effective when multiple batches are released together. |
| Quality Assurance | Provides detailed batch-specific quality data. | Ensures overall lot quality but may miss individual batch variability. |
Introduction to Batch and Lot Release Testing in Medical Devices
Batch release testing in medical devices involves assessing a specific quantity of products manufactured under uniform conditions to ensure safety and performance before market distribution. Lot release testing refers to the evaluation of a collection of batches produced within a defined time frame or production cycle, providing broader quality assurance across multiple production runs. Both testing methods are critical for regulatory compliance, risk management, and maintaining consistent device reliability.
Defining Batch Release Testing: Purpose and Process
Batch release testing in medical devices ensures each manufactured batch meets predefined quality standards, confirming safety and efficacy before market distribution. The process involves rigorous analytical and functional evaluations, verifying compliance with regulatory requirements and specifications. This testing safeguards product consistency, identifies defects early, and prevents substandard devices from reaching patients.
What is Lot Release Testing? Key Differences
Lot release testing refers to the quality control process conducted on a specific batch or lot of a medical device to ensure it meets predefined safety and performance standards before being distributed. Unlike batch release testing, which typically evaluates individual production batches, lot release testing often involves assessing a representative sample from the entire lot, focusing on regulatory compliance and consistency across multiple batches. Key differences include the scope of testing, sample size, and regulatory emphasis, with lot release testing providing a broader assurance of uniform product quality and safety.
Regulatory Requirements for Batch vs Lot Release Testing
Regulatory requirements for batch release testing mandate thorough quality control analysis of each individual batch to ensure compliance with safety and efficacy standards before market approval. Lot release testing regulations often pertain to specific product categories, requiring verification of consistency and potency across multiple production lots to satisfy regulatory agencies. Both testing protocols are crucial for maintaining product integrity but differ in scope and frequency based on defined regulatory frameworks.
Quality Control Parameters in Batch and Lot Release
Batch release testing in medical devices involves comprehensive Quality Control (QC) parameters, including physical, chemical, and microbiological assessments to ensure consistency and compliance with regulatory standards. Lot release testing focuses on verifying critical attributes such as sterility, functionality, and packaging integrity to confirm that each produced lot meets predefined specifications. Both processes emphasize stringent QC measures to maintain device safety, efficacy, and quality before market distribution.
Documentation and Record Keeping for Release Testing
Batch release testing requires comprehensive documentation, including detailed test results, specifications, and compliance certificates, to ensure traceability and regulatory adherence. Lot release testing emphasizes maintaining consistent records of sample selection, testing procedures, and acceptance criteria for each lot to support quality assurance and audit readiness. Precise and structured record keeping in both processes is critical for meeting FDA, EMA, and ISO standards.
Risk Management in Batch and Lot Release Testing
Batch release testing focuses on evaluating the quality and safety of individual production batches through comprehensive analytical and functional assays, ensuring each batch meets strict regulatory specifications before distribution. Lot release testing, often involving selected samples from a production lot, incorporates risk management strategies by emphasizing critical quality attributes and potential variability within the lot, thereby optimizing resource allocation and enhancing patient safety. Effective risk management in both batch and lot release testing minimizes the likelihood of defective medical devices reaching the market and supports compliance with regulatory frameworks such as ISO 13485 and FDA QSR.
Compliance Challenges: Batch vs Lot Testing
Batch release testing ensures each individual batch of a medical device meets regulatory specifications, addressing variability in production processes and maintaining product safety. Lot release testing evaluates a defined quantity or group of products simultaneously, which can present challenges in detecting variations within the lot that may impact compliance. Navigating regulatory requirements for both testing methods demands rigorous documentation and validation to mitigate risks associated with quality inconsistency and ensure adherence to standards like FDA and ISO 13485.
Impact on Product Quality and Patient Safety
Batch release testing directly assesses the quality and safety of each produced batch, ensuring compliance with regulatory standards and minimizing the risk of defective products reaching patients. Lot release testing often involves a representative sample evaluation, which can introduce variability in detecting potential quality issues, impacting consistent product safety assurances. Rigorous batch release testing enhances traceability and control over manufacturing processes, significantly reducing patient risk and improving overall therapeutic efficacy.
Future Trends in Release Testing for Medical Devices
Future trends in release testing for medical devices emphasize increased automation and digital integration to enhance accuracy and efficiency in batch release testing. Advanced analytics and real-time data monitoring are expected to enable more predictive lot release testing, reducing delays and improving compliance with regulatory standards. The adoption of innovative technologies such as AI-driven quality control and blockchain for traceability will drive significant improvements in transparency and reliability across the medical device supply chain.
Batch release testing vs Lot release testing Infographic
 productdif.com
productdif.com